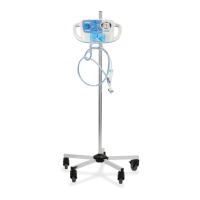
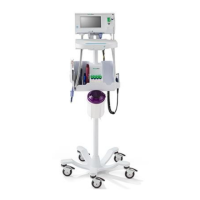
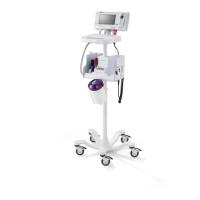
What to do if the nebulizer is not aerosolizing correctly on my Hillrom Medical Equipment?
- MmartinezpaulAug 5, 2025
If the nebulizer on your Hillrom medical equipment isn't aerosolizing correctly, first, ensure it's properly connected. If it is, the nebulizer may be dirty. Clean or replace it, referring to the nebulizer package for cleaning instructions.