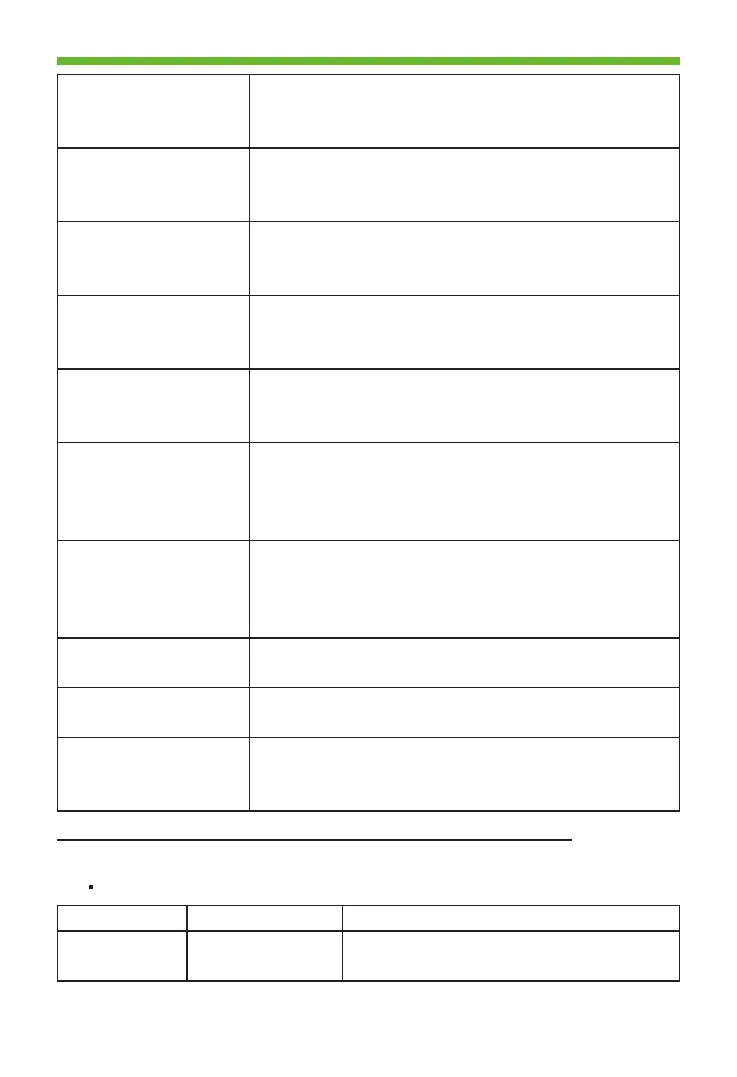
28
Digital Intra-oral X-Ray Imaging System Pluto0001X /Pluto0002X User's Manual
IEC 60601-2-
65:2012+A1:2017
Medical electrical equipment - Part 2-65: Particular
requirements for the basic safety and essential
performance of dental intra-oral X-ray equipment
IEC 60601-1-
6:2010+A1:2013
Medical electrical equipment Part 1-6: General
requirements for basic safety and essential
performance — Collateral standard: Usability
CAN/CSA-C22.2
NO. 60601-1-
6:11+A1:2015
Medical electrical equipment Part 1-6: General
requirements for basic safety and essential
performance — Collateral standard: Usability
KS C IEC 60601-1-
6:2011
Medical electrical equipment Part 1-6: General
requirements for basic safety and essential
performance — Collateral standard: Usability
EN 60601-1-
6:2010+A1:2015
Medical electrical equipment Part 1-6: General
requirements for basic safety and essential
performance — Collateral standard: Usability
IEC 60601-1-2:2014 Medical electrical equipment – Part 1-2: General
requirements for basic safety and essential
performance– Collateral standard: Electromagnetic
disturbances– Requirements and tests
EN 60601-1-2:2015 Medical electrical equipment – Part 1-2: General
requirements for basic safety and essential
performance– Collateral standard: Electromagnetic
disturbances– Requirements and tests
EN 62304:2006/
AC:2008
Medical device software – Software life-cycle
processes
EN 62366:2008 Medical devices – Application of usability
engineering to medical devices
ISO 15223-1:2016 Medical devices-symbols to be used with medical
device labels, labeling and information to be
supplied–Part1:General requirements
5.2 Guidance and manufacture’s declaration for EMC
5.2.1 EMI Compliance Table
Emissions
Phenomenon Compliance Electromagnetic environment
RF emissions CISPR 11
Group 1, ClassB
Professional healthcare facility
environment
 Loading...
Loading...