22
|
Arm-type Fully Automatic Blood Pressure Monitor DBP-6191
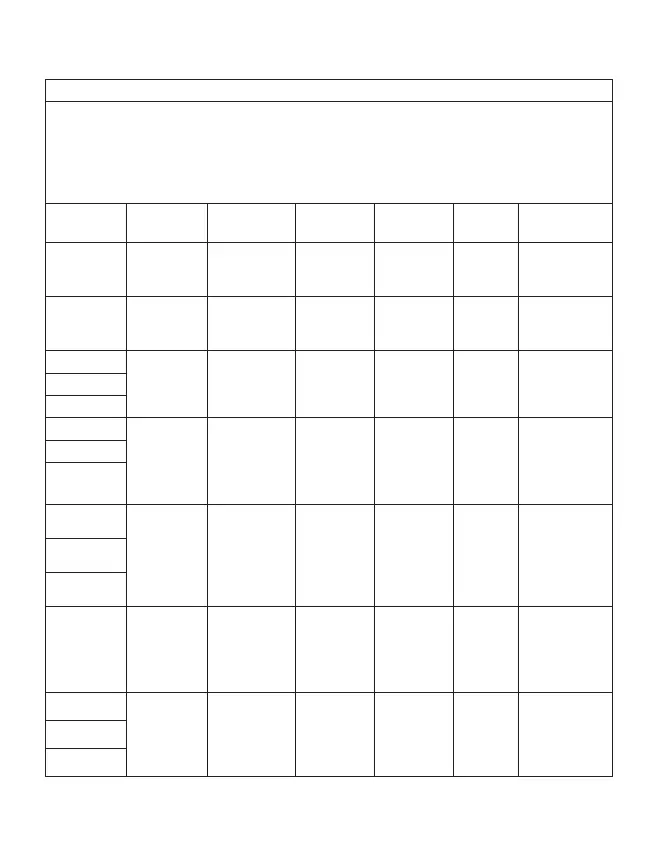
Table 3
Guidance and declaration of manufacturer-electromagnetic immunity
Nowadays, many RF wireless equipments have being used in various healthcare locations where medical equipment
and/or systems are used. When they are used in close proximity to medical equipment and/or systems, the medical
equipment and/or systems’ basic safety and essential performance may be affected. Arm-type Fully Automatic
Digital Blood Pressure Monitor has been tested with the immunity test level in the below table and meet the related
requirements of IEC 60601-1-2:2014. The customer and/or user should help keep a minimum distance between
RF wireless communications equipment and this medical equipment and/or systems as recommended below.
Test frequency
(MHz)
Band (MHz) Service Modulation Maximum
power (W)
Distance
(m)
Immunity
test level (V/m)
385 380–390 TETRA 400 Pulse
modulation
18Hz
1,8 0,3 27
450 430–470 GMRS 460
FRS 460
FM ±5 kHz
deviation 1
kHz sine
2 0,3 28
710 704–787 LTE Band
13, 17
Pulse
modulation
217Hz
0,2 0,3 9
745
780
810 800–960 GSM 800/900,
TETRA 800,
iDEN 820,
CDMA 850,
LTE Band 5
Pulse
modulation
18Hz
2 0,3 28
870
930
1720 1700–1990 GSM 1800;
CDMA 1900;
GSM 1900;
DECT; LTE Band
1, 3, 4, 25;
UMTS
Pulse
modulation
217 Hz
2 0,3 28
1845
1970
2450 2400–2570 Bluetooth,
WLAN,
802.11 b/g/n,
RFID 2450, LTE
Band 7
Pulse
modulation
217Hz
2 0,3 28
5240 5100–5800 WLAN 802.11
a/n
Pulse
modulation
217Hz
0,2 0,3 9
5500
5785
 Loading...
Loading...