TRUE METRIX
®
PRO System Comprehensive Resource Guide (GDH-FAD Enzyme) Quality Control Testing Data Form 22
Quality Control Testing
Data Form (located in Appendix)
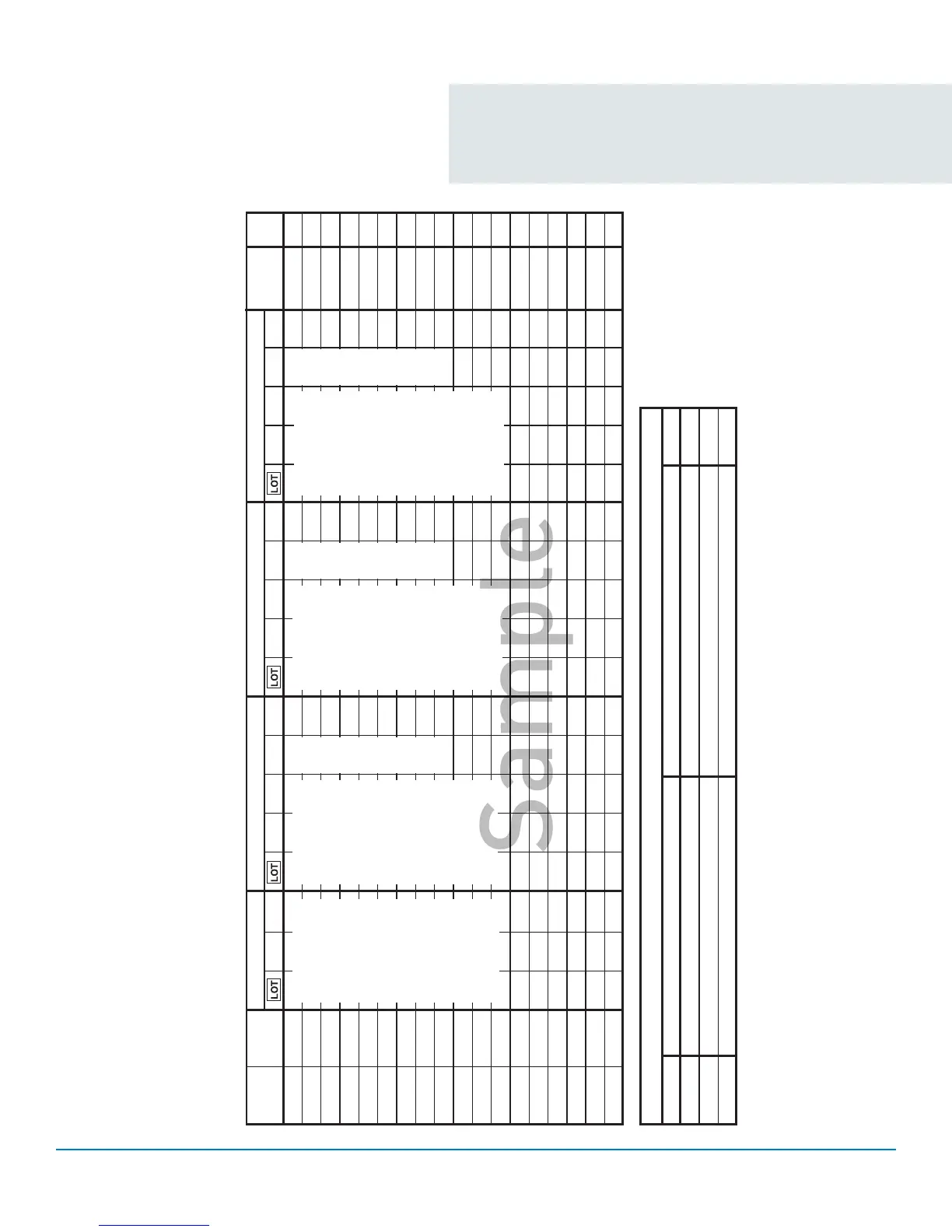
QUALITY CONTROL TESTING DATA FORM
Meter Serial Number _________________
(see number on Meter label below the bar code)
*Note any problems in Troubleshooting section below.
Test Strips Control Solution - Level __________
Control Solution - Level __________
Date Time
Date
Opened
Date
Opened
Acceptable
Range
Date
Opened
Acceptable
Range
Result Result
Initials
*
*TROUBLESHOOTING
Date Problem
Action
Initials
On TRUE
METRIX
®
PRO
Test Strip vial label,
write date vial
opened. Discard
vial if either
4 months after
opening or
EXP date printed
on the vial label has
passed, whichever
comes first.
On
TRUE
METRIX
®
PRO
Control Solution
bottle label, write
date bottle opened.
Discard bottle if
either 3 months
after opening or
after EXP date
printed on the
bottle label has
passed, whichever
comes first.
On
TRUE
METRIX
®
PRO
Control Solution
bottle label, write
date bottle opened.
Discard bottle if
either 3 months
after opening or
after EXP date
printed on the
bottle label has
passed, whichever
comes first.
Printed
on
vial
label
of
test
strips
being
used.
Printed
on
vial
label
of
test
strips
being
used.
Control Solution - Level __________
Date
Opened
Acceptable
Range
Result
Printed
on
vial
label
of
test
strips
being
used.
On
TRUE
METRIX
®
PRO
Control Solution
bottle label, write
date bottle opened.
Discard bottle if
either 3 months
after opening or
after EXP date
printed on the
bottle label has
passed, whichever
comes first.
EXP
EXP
EXP
EXP
 Loading...
Loading...