Nox A1 Manual
~ 41 ~
Regulatory Information
Performance Testing and Validation Summary
The Nox Sleep System has been tested and verified in various phases to include internal testing,
verification, and validation as well as external testing to assure product safety, effectiveness, and
reliability. The design was verified and validated, including clinical evaluation, throughout the design
process, according to requirement specifications and intended use. External accredited test houses
were used to conduct testing needed to comply with the applicable standards regarding
Electromagnetic Compatibility (EMC) and patient safety as well as additional RF testing to assure
compliance with Federal Communications Commission (FCC) and Radio and Telecommunication
Terminal Equipment (R&TTE) Directive.
The compliance of the Nox Sleep System towards patient safety and medical device standards has
ONLY been verified and validated with the sensors and accessories listed in this manual. This includes
all signal characteristics and automatic analysis provided by the Nox Sleep System.
Furthermore, use of other sensors or accessories with the Nox A1 recorder invalidates the Declaration
of Conformity issued by Nox Medical towards the Medical Devices Directive 93/42/EEC (MDD). Use of
other components than verified, validated or recommended by Nox Medical with the Nox A1 recorder
is considered to be a modification of the Nox Sleep System. Such modifications could result in the
system not performing as intended and cause serious harm to the patient.
Nox Medical holds an ISO 13485:2016 certified Quality Management System which complies with the
requirements of the Medical Device Directive (MDD), FDA Quality System Regulation (QSR) and
Canada Medical Device Regulations (CMDR).
Nox A1 Classifications
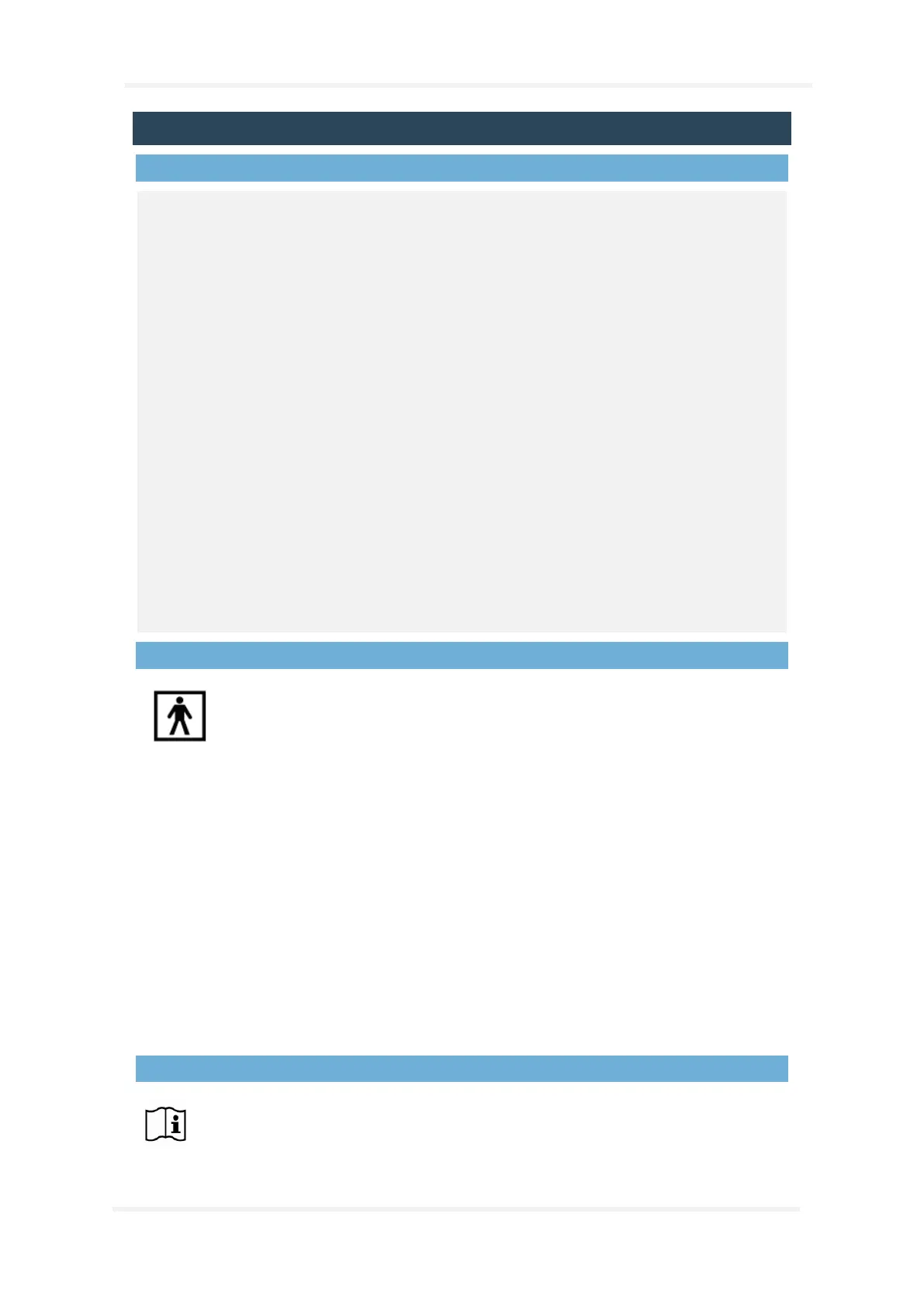
Degree of protection (applied part) against electric shock: The entire device is an
applied part and is classified as of type BF (see symbol to the left).
Powering of the device: The device is internally powered.
Degree of protection against harmful ingress of liquids and particulate matter:
o The Nox A1 recorder is classified IP20, i.e. as defined by the
standard IEC 60529 it is protected against solid foreign objects of
12.5 mm diameter and greater, but it is not protected against
harmful ingress of liquids.
Method of sterilization: The device is NOT delivered sterile or intended to be
sterilized.
Suitability for use in an oxygen rich environment: The device is NOT intended for
use in an oxygen rich environment.
Suitability for use with flammable agents and anesthetics: The device is NOT
intended for use in conjunction with flammable agents or with flammable
anesthetic mixture with air or with oxygen or nitrous oxide.
Mode of operation: The device is intended for continuous operation.

 Loading...
Loading...