6
Intended use
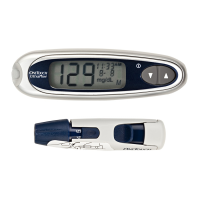
The OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System is intended to be used for the quantitative
measurement of glucose (sugar) in fresh capillary whole
blood samples drawn from the fingertip. The system is
intended to be used by a single patient and should not be
shared.
The OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System is intended for self-testing outside the body (in
vitro diagnostic use) by people with diabetes at home and
with their healthcare professionals in a clinical setting as
an aid to monitor the effectiveness of diabetes control.
The OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System is not to be used for the diagnosis of or screening
of diabetes or for neonatal use.
The OneTouchSelect Plus Flex™ Blood Glucose Monitoring
System is not for use on critically ill patients, patients in
shock, dehydrated patients or hyperosmolar patients.
in
PF3130451Rev1_OTSPF_OB_I_GB_en_zug_R2.indd 6 3/12/15 11:35 AM
Statement of Use: Verify status before each use
Effectivity Date: Mar 18 2015 Status: Current State: Released
Type: Project File Name: 3130451 Revision: 1
 Loading...
Loading...