English 1
ENGLISH
Welcome
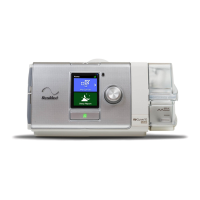
The AirCurve
™
10 VAuto, AirCurve 10 S and AirCurve 10 ST are bilevel positive airway pressure
devices.
WARNING
Read this entire guide before using the device.
CAUTION
In the US, Federal law restricts this device to sale by or on the order of a physician.
Indications for use
AirCurve 10 VAuto
The AirCurve 10 VAuto device is indicated for the treatment of obstructive sleep apnea (OSA) in
patients weighing more than 66 lb (30 kg). It is intended for home and hospital use.
The humidifier is intended for single patient use in the home environment and re-use in a
hospital/institutional environment.
AirCurve 10 S
The AirCurve 10 S device is indicated for the treatment of obstructive sleep apnea (OSA) in patients
weighing more than 66 lb (30 kg). It is intended for home and hospital use.
The humidifier is intended for single patient use in the home environment and re-use in a
hospital/institutional environment.
AirCurve 10 ST
The AirCurve 10 ST device is indicated for the treatment of obstructive sleep apnea (OSA) in
patients weighing more than 66 lb (30 kg). It is intended for home and hospital use.
The humidifier is intended for single patient use in the home environment and re-use in a
hospital/institutional environment.
Contraindications
Positive airway pressure therapy may be contraindicated in some patients with the following pre-
existing conditions:
x severe bullous lung disease
x pneumothorax or pneumomediastinum
x pathologically low blood pressure, particularly if associated with intravascular volume depletion
x dehydration
x cerebrospinal fluid leak, recent cranial surgery, or trauma.
Adverse effects
You should report unusual chest pain, severe headache, or increased breathlessness to your
prescribing physician. An acute upper respiratory tract infection may require temporary
discontinuation of treatment.
The following side effects may arise during the course of therapy with the device:
x drying of the nose, mouth, or throat
x nosebleed
x bloating

 Loading...
Loading...