Event traceability
Considering the critical role of endoscopic devices in an infection control process, it is necessary to be able to
provide evidence of conformity with the relevant standards for events occurring individually on each
endoscope having passed through the aseptic storage unit.
In a ranked and operational manner, a ticket is produced in a single or duplicate copy when each endoscope is
removed. The information provided includes the identification of the operator inserting and removing the
endoscope, the date and time of entry and removal as well as conformity with the recommendations or, where
appropriate, of non-conformity including the main reason for non-conformity.
A printer returns recorded parameters for insertion, storage and removal of each endoscope. These
parameters are also saved on a memory card installed on the back of the control panel.
CONFIGURATION OF THE STORAGE UNIT
The storage unit circulates a constantly renewed flow of filtered, optionally heated air, distributed using a
unidirectional vertical piston in the endoscope storage zone. This area comprises a horizontal motorised plate
supporting a maximum of 10 endoscopes suspended vertically.
The storage area is accessed via a door providing access to only one free space per endoscope (when inserting)
or one endoscope (the one selected for removal). The relevant space is presented to the operator by the
motorized rotation of the rack plate.
The plate receives all 10 functions associated with the principal blowing cycle for endoscope channels by
means of connection to a local medical grade compressed air distribution network, that is, the couplings of
each section and the continuous control of the presence of the coupling.
The aerodynamic unit maintains the endoscopes in a controlled environment using a set of upstream filters
controlled at the time of use and a high-efficiency downstream filter which is controlled against clogging using
a differential pressure switch.
All parameters, functions and cycles are monitored and controlled automatically.
Operation tasks are managed using a touch screen with dedicated functions which does not require calibration
by the operator except for installation and commissioning on site.
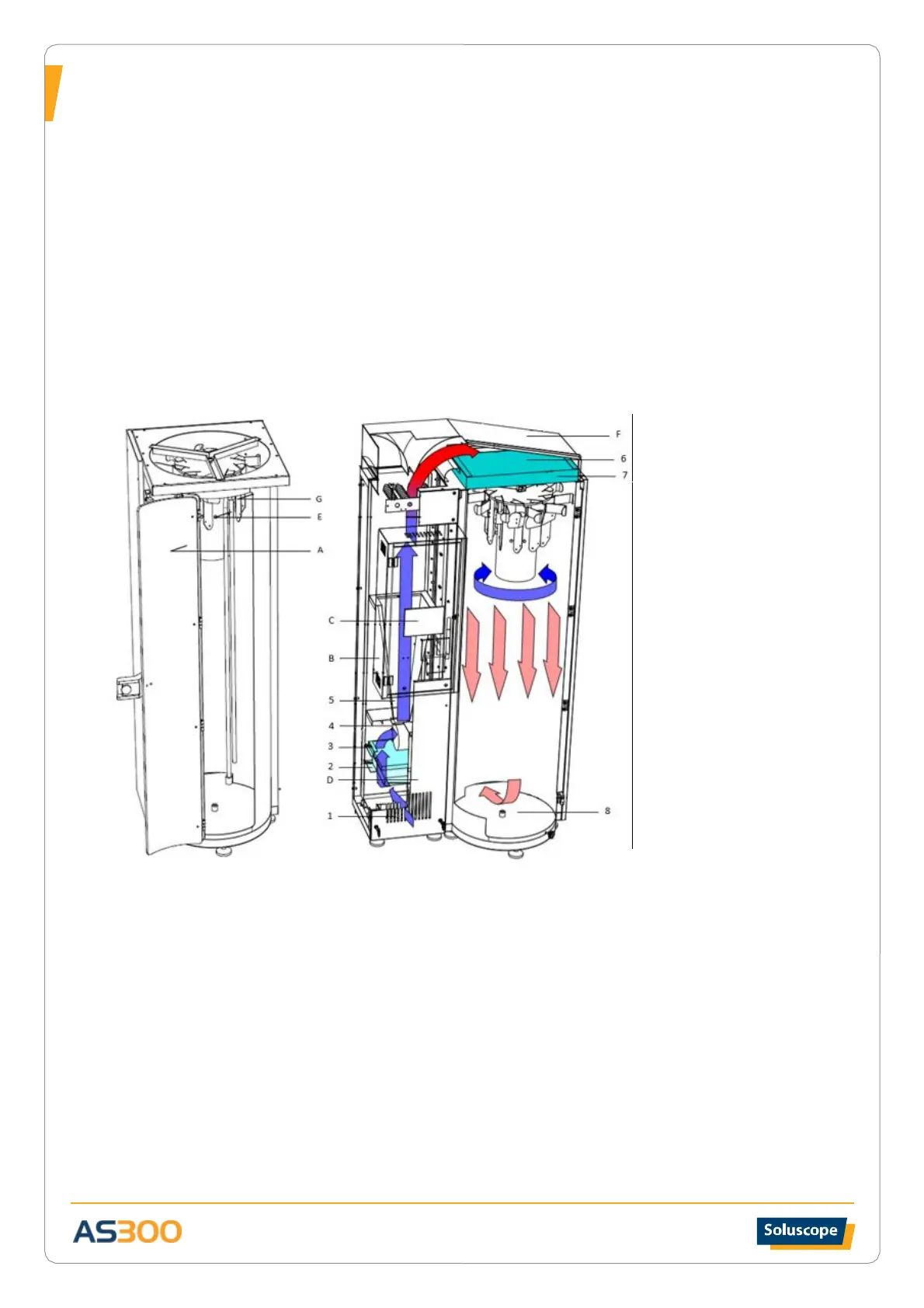
STORAGE UNIT
A – Transparent doors
B – Electrical cabinet
C – Touch screen
D – Door of the filter
compartment
E – Compressed medical air
connector
F – Filter cabinet H14
(single or double)
G – Rotating endoscope rack
AIR TREATMENT CIRCUIT
1 – Fresh air entry
2 – Pre-filter for fresh air EU 3
(80% gravimetric)
3 – Filtration EU 5
(95% gravimetric)
4 – Centrifugal variable output
ventilator
5 – Flow stabilisation cone
6 – Air distribution cabinet
7 – High-efficiency H14 filter
(99.99 DOP for particles of 0.3μ)
8 – Air extractor
 Loading...
Loading...