22
nitions
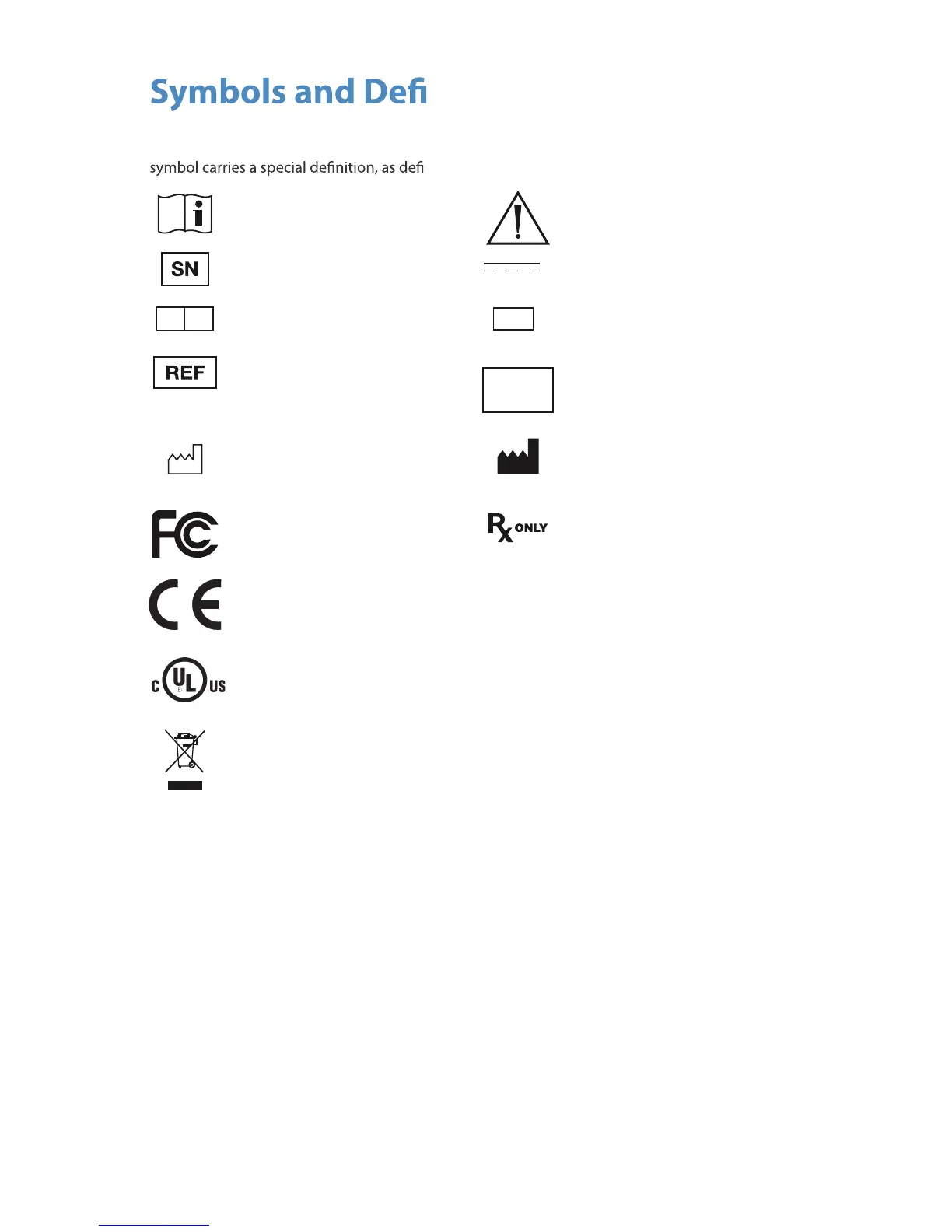
The following symbols appear on the product, its labeling, or the product packaging. Each
ned below:
Consult Instructions for Use
Attention: See Instructions for Use
Serial Number Direct Current
EC REP
Authorized European
Representative
QTY
Quantity
Catalog number
Made in
Korea
Country of Origin
Date of Manufacture Legal manufacturer
Tested to comply with FCC
Class B standards
Federal law restricts this device
to sale by or on the order of a
physician
Complies with the
requirements of directive
93/42/EEC
This p
Medical Equipment is in accordance with UL 60601-1 and CAN/CSA C22.2 No. 601
in regards to electric shock, re hazards, and mechanical hazards.
roduct contains electrical waste or electronic equipment. It must not be
disposed of as unsorted municipal waste and must be collected separately.
IP23
Degrees of protection against the
ingress of water
 Loading...
Loading...