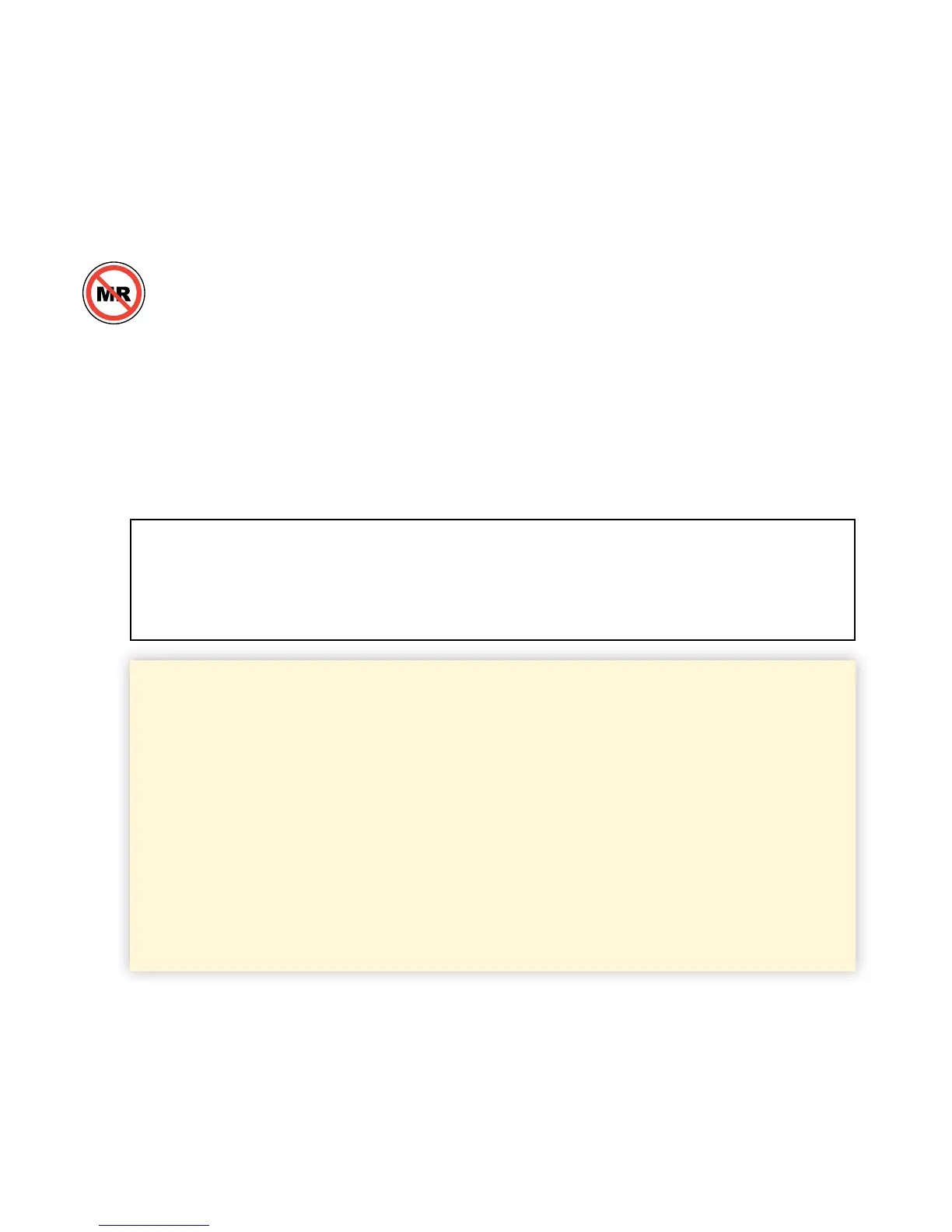
Contraindications
The FreeStyle Libre Pro Flash Glucose Monitoring System
must be removed prior to Magnetic Resonance Imaging (MRI),
Computed Tomography (CT) scan, or high-frequency electrical
heat (diathermy) treatment. The eect of MRI, CT scans, or
diathermy on the performance of the System has not been
evaluated. The exposure may damage the Sensor and may
impact proper function of the device to detect trends and track
patterns in the user’s glucose values during the wear period.
WARNING: The FreeStyle Libre Pro Flash Glucose Monitoring
System contains small parts that may be dangerous if
swallowed.
CAUTION:
• Performance of the System when used with other implanted
medical devices, such as pacemakers, has not been evaluated.
• Some individuals may be sensitive to the adhesive that
keeps the Sensor attached to the skin. If your patient notices
signicant skin irritation around or under their Sensor, they
should remove the Sensor and stop using the FreeStyle Libre
Pro System. Follow your facility’s procedures for handling
skin reactions.
3
 Loading...
Loading...