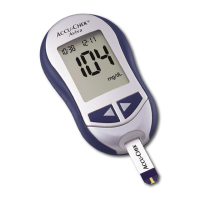
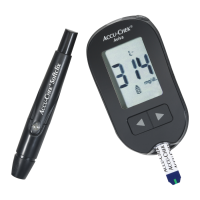
Please consult instructions for use
Caution (refer to accompanying documents).
Please refer to safety-related notes in the
manual accompanying this instrument.
Store at
Use by / Expiry date
unopened/ opened
Discard three months after opening or by the
expiry date, whichever comes first.
Manufactured by
Catalogue number
Lot number
Listed by Underwriter’s
Laboratories, Inc.© in
accordance with UL 61010A-1
and CAN/CSA C22.2 No.1010-1.
For in vitro diagnostic use.
This product fulfils the requirements of
Directive 98/79/EC on in vitro diagnostic
medical devices.
67
 Loading...
Loading...