119
Technical Information
10
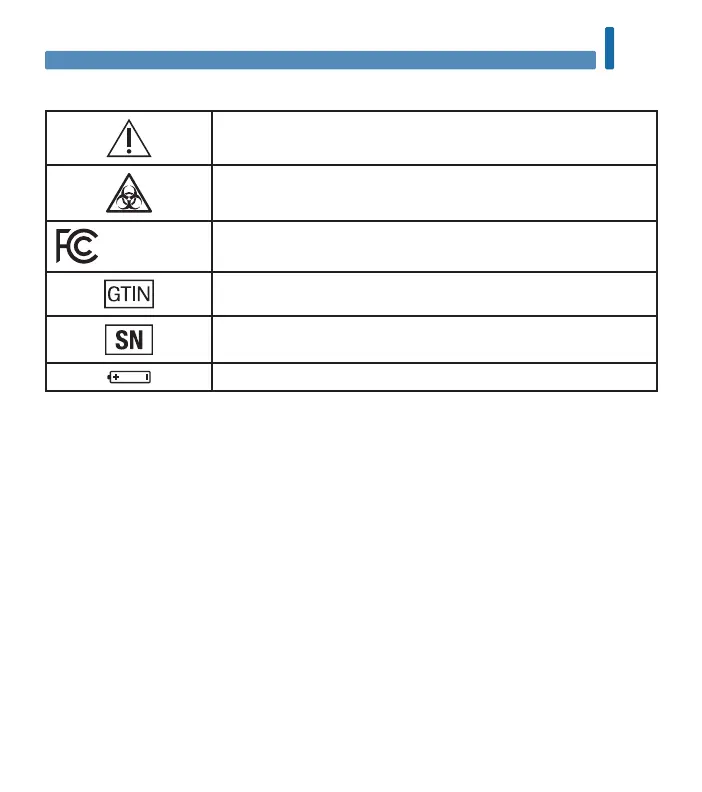
Explanation of Symbols
Caution, refer to safety‑related notes in the instructions for use
accompanying this product.
Biological Risks – used meters carry a risk of infection.
FCC ID: WX3‑126
This device complies with Part 15 of the FCC Rules.
Global Trade Item Number
Serial number
3‑volt coin cell type CR2032
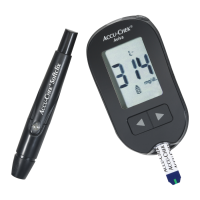
Additional Supplies
Test Strips: Accu‑Chek Guide test strips
Control Solutions: Accu‑Chek Guide control solutions
Lancets: Accu‑Chek FastClix 102‑ct. lancet drums (17‑6 ct. drums)
References
1
FDA Public Health Notification: “Use of Fingerstick Devices on More than One Person Poses Risk for
Transmitting Bloodborne Pathogens: Initial Communication, (2010). Update 11/29/2010.”
http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm224025.htm. Accessed June 1, 2016.
2
CDC Clinical Reminder: “Use of Fingerstick Devices on More than One Person Poses Risk for Transmitting
Bloodborne Pathogens, (2010).” http://www.cdc.gov/injectionsafety/Fingerstick‑DevicesBGM.html.
Accessed June 1, 2016.
3
Healthcare Infection Control Practices Advisory Committee (HICPAC), William A. Rutala, Ph.D., M.P.H.,
and David J. Weber, M.D., M.P.H. Centers for Disease Control and Prevention, 2008. “Guideline for
Disinfection and Sterilization in Healthcare Facilities.” Atlanta.
http://www.cdc.gov/hicpac/pdf/guidelines/Disinfection_Nov_2008.pdf. Accessed June 1, 2016.
54988_08100918001_EN.indb 119 9/30/16 5:04 PM
 Loading...
Loading...