9
USA
7.8.2 Mechanical alkaline cleaning and thermal disinfection
Machine type: single-chamber cleaning/disinfection device without ultrasound
D-W: Drinking water
FD–W: Fully desalinated water (demineralized, low microbiological contamination: drinking water quality at least)
*Recommended: BBraun Helimatic Cleaner alkaline
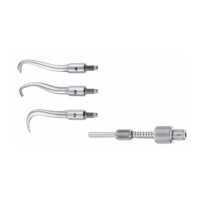
► Insert product 1 in the correct position in the Aesculap ECCOS holder
systems, see Fig. B.
► Attach the rinsing adapter 14 (GA344211) to the product 1 in the
mount 11.
► Then connect the interior rinse device and connect to the rinse connec-
tor of the cleaning/disinfection device/rinse cart.
► Attach the Kirschner wire protection sleeve 12 to a rinse hose.
► After automatic cleaning/disinfection, check visible surfaces for resi-
dues and repeat the cleaning/disinfection process as needed.
7.9 Inspection, maintenance and checks
► Allow the product to cool down to room temperature.
► Spray product after every cleaning and disinfection with Aesculap
STERILIT Power Systems oil spray GB600 using the oil spray adapter 15
GB600880 (green) for approx. 2 s, see Fig. A.
Note
Aesculap additionally recommends the occasional spraying of moving parts
(such as knobs, couplings) with the Aesculap STERILIT Power Systems oil
spray.
► Check the product after each cleaning and disinfection for the follow-
ing: cleanliness, damage, function, abnormal operation noise, excessive
heat or heavy vibration.
► Set aside the product if it is damaged.
7.10 Packaging
► Follow the instructions for use of the respective packaging and holders
(e.g. instructions for use TA009721 for Aesculap ECCOS holder sys-
tems).
► Insert products in the correct position into the Aesculap ECCOS holder
system, see Fig. B.
► Pack trays appropriately for the sterilization process (e.g. in Aesculap
sterile containers).
► Ensure that the packaging will prevent a recontamination of the pro-
duct.
7.11 Steam sterilization
Note
Remove all attached components from the product (tools, accessories)
before sterilization.
► Make certain that all external and internal surfaces of the product will
be exposed to the sterilizing agent.
► Use a validated sterilization method:
– Steam sterilization using fractional vacuum process
– Steam sterilizer DIN EN 285 and validated pursuant to
DIN EN ISO 17665
– Sterilization using fractionated vacuum process at 134 °C/holding
time 5 min
When sterilizing multiple products in one steam sterilizer:
► Ensure that the maximum permitted load specified by the manufac-
turer for the steam sterilizer is not exceeded.
7.12 Sterilization for the US market
■ Aesculap advises against sterilizing the device by flash sterilization or
chemical sterilization.
■ Sterilization may be accomplished by a standard prevacuum cycle in a
steam autoclave.
To achieve a sterility assurance level of 10
-6
, Aesculap recommends the
following parameters:
*Aesculap has validated the above sterilization cycle and has the data on
file. The validation was accomplished in an Aesculap sterile container
cleared by FDA for the sterilization and storage of these products. Other
sterilization cycles may also be suitable, however individuals or hospitals
not using the recommended method are advised to validate any alterna-
tive method using appropriate laboratory techniques. Use an FDA cleared
accessory to maintain sterility after processing, such as a wrap, pouch, etc.
Phase Step T
[°C/°F]
t
[min]
Water
quality
Chemistry/Note
I Pre-rinse <25/77 3 D–W -
II Cleaning 55/131 10 FD–W
■ Concentrate, alkaline:
–pH ~ 13
– <5 % anionic surfactant
■ 0.5 % working solution
– pH ~ 11*
III Intermediate rinse >10/50 1 FD–W -
IV Thermal disinfection 90/194 5 FD–W -
V Drying - - - at least 10min at max. 120°C
Aesculap Orga Tray/Sterile container (perforated bottom)
Minimum cycle parameters*
Sterilization
method
Temp. Time Minimum drying time
Prevacuum 270 °F/275 °F 4 min 20 min
Document No.: TA014436 - Version: 1.0 - Document ID: SOP-AIC-5002244 Date/Time Printed/Viewed: 2022-04-13 16:01 (CET)
Effective

 Loading...
Loading...