RSR-000886-000 (1) Page 30 of 40
6. Quality and Regulatory
6.1. Quality Systems
The FXi was designed, developed, and manufactured in accordance with ISO13485:2016 –
Medical Devices – Quality management systems.
6.2. Device Classification
The FXi complies with 21 CFR Chapter 1, Sub chapter J, as administered by the Center for
Devices and Radiological Health (CDRH) of the US Food and Drug Administration (FDA).
According to the applicable standards, the laser system is classified as follows:
• Class I Type B device per EN/IEC 60601-1
• Class 4 laser product according to IEC 60825-1
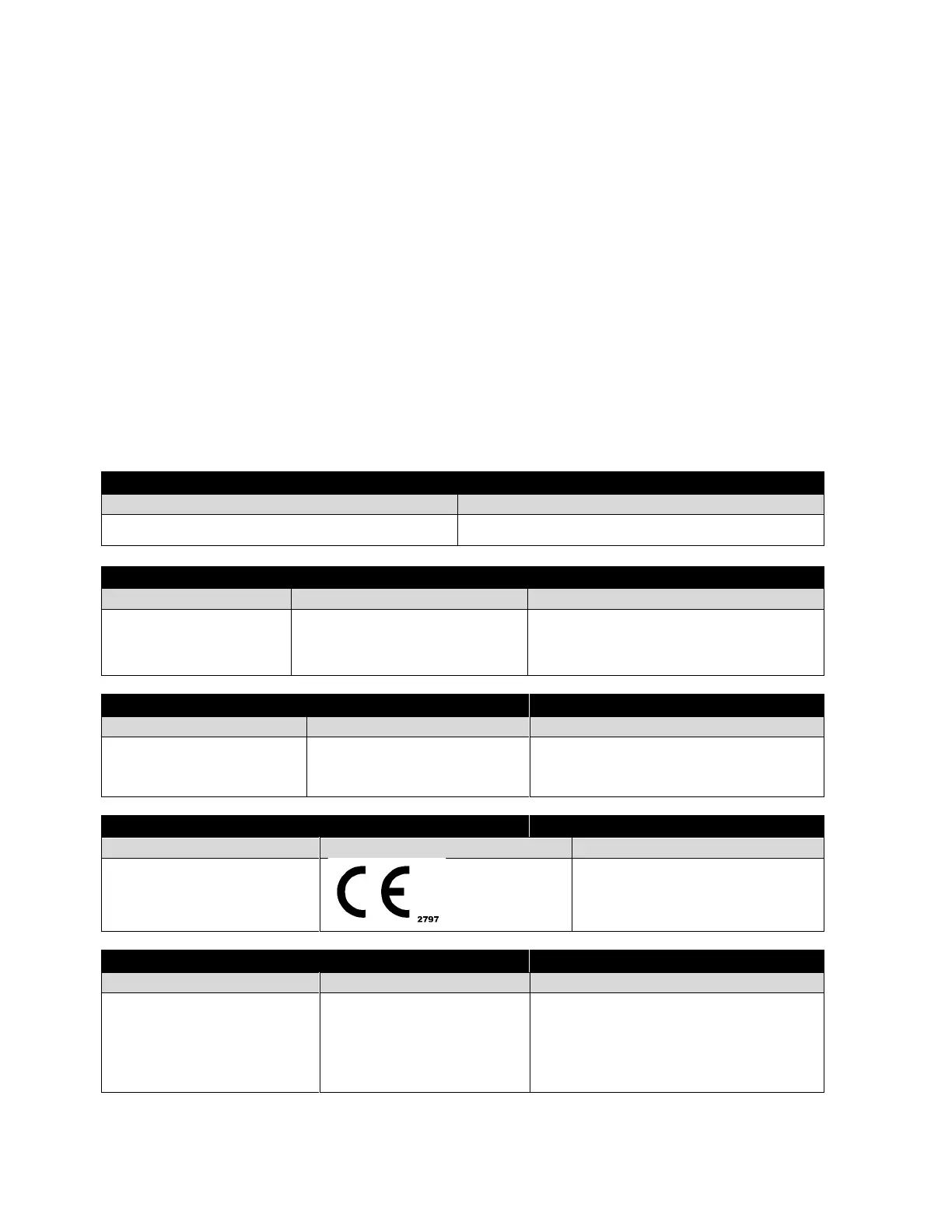
6.3. Declaration of Conformity (EU)
LightForce
®
Therapy Lasers
LTC-1510-L-B6 LightForce
®
FXi
101 Lukens Drive, Suite A
New Castle, DE 19720
USA
Quality & Regulatory Director
AUTHORIZED REPRESENTATIVE
Prinsessegracht 20
2514 AP The Hague
The Netherlands
+31.70.345.8570 - phone
+31.70.346.7299 - fax
europe@emergogroup.com
Annex II of MDD
93/42/EEC Council
Directive
IEC 60601-1:2005+A1:2012
IEC 60601-1-2:2015
IEC 60601-1-6:2010+A1:2013
IEC 60601-2-22:2007+A1:2013
IEC 60825-1:2014
LiteCure, LLC declares that the above-mentioned product meets the fundamental requirements of the applied standards
and the Medical Device Directive 93/42/EEC+2007/47/EEC as transposed in the national laws of the Member States.

 Loading...
Loading...