Rinse the test cell with unused DI water several times after each measurement, shaking the water
out after each rinse.
Protective Cap
PosiTector SST probe is shipped with a protective cap over the probe. Remove this cap prior to
use. Replace the cap to protect the indentor and presser foot when not in use.
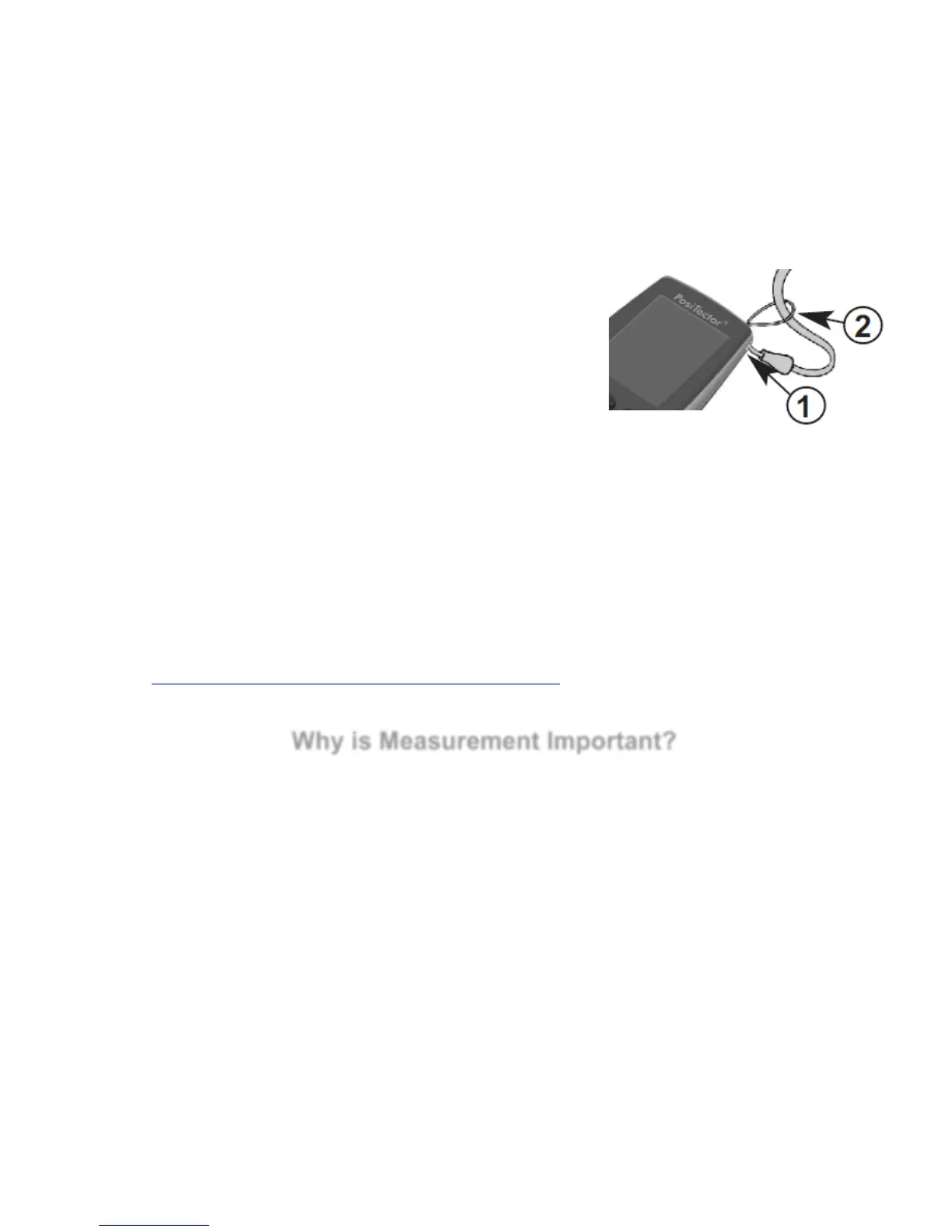
Wrist Strap
We recommend attaching and wearing the supplied wrist strap.
Protective Lens Shield
The Gage display lens is covered with a thin plastic film for
protection against fingerprints and other marks during
shipment. This film, while usually removed before using the
instrument, can be left in place if desired. Replacements can be purchased.
Certification
All PosiTector SST probe includes a Certificate of Calibration. For organizations with
re-certification requirements, instruments may be returned at regular intervals for calibration.
DeFelsko recommends that customers establish calibration intervals based upon their own
experience and work environment.
Based on our product knowledge, data and customer feedback, a one year calibration interval
from either the date of calibration, date of purchase, or date of receipt is a typical starting point.
Written calibration procedures are available from DeFelsko Corporation at no charge.
See: www.defelsko.com/quality/calibration_procedures.htm
Why is Measurement Important?
Before the application of protective coatings, steel substrates must be carefully cleaned. The
surface should then be tested to determine if the level of salt contamination is acceptable or not.
Soluble salts are not visible to the naked eye. If allowed to remain on the substrate in sufficient
quantities, they can draw moisture through the coating and cause premature coating failure from
osmotic blistering or disbondment.
The test for water-soluble salts is performed in accordance with ISO 8502-9. As a part of that
method, the extraction of soluble salt contaminants for analysis is performed in accordance with
ISO 8502-6, commonly known as the Bresle Method.
Put simply, the Bresle Method involves placing a volume of water in a chamber against the steel
surface. This water dissolves soluble salts present on the surface, raising the conductivity of the
water. The measured increase in conductivity establishes the concentration of soluble salts.

 Loading...
Loading...