Do you have a question about the Dynatronics Dynatron 850 Plus and is the answer not in the manual?
Overview of the Dynatron 850plus and 550plus devices, highlighting features and capabilities.
Details on features added to models with software Rev. 1.01 or later.
Description of the various therapeutic modalities offered by the devices.
Explanation of the user-friendly treatment setup process.
Guidance on the manual's organization and how to find information.
Instructions for unpacking the device and checking for damage.
List of accessories included with the Dynatron 850plus or 550plus units.
Information on available soundhead sizes and optional probes.
List of optional and replacement accessories available for purchase.
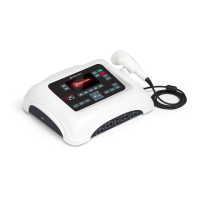
Detailed description of the device's physical features and control panel.
Guidance on using toggle keys for making selections within the device.
Explanation of the Time and Power-Intensity displays and indicators.
Details on the optional probes for microcurrent treatment.
Information about the optional high volt probe for attended treatments.
A summary of special key press combinations and their results.
Basic guidelines for powering the device on/off and setting up treatments.
Step-by-step guide to setting up a basic treatment.
Explanation of the conductance bar graph feature for IFC, Premod, and Microcurrent.
Information on the safety feature warning about open circuits during IFC setup.
Setup and operation instructions for Interferential and Premodulated therapy.
Setup and operation instructions for Biphasic and Russian stimulation.
Setup and operation instructions for High Volt therapy.
Setup and operation instructions for Microcurrent therapy.
Setup and operation instructions for Ultrasound therapy (850plus only).
Instructions for setting up and performing combination therapy.
Procedure for saving custom treatment parameters as new defaults.
Instructions to reset all saved defaults to factory settings.
Lists of default settings for various modalities.
Specifications for the optional battery pack and adapter cord.
Factors affecting the duration of battery-powered operation.
General information on Interferential and Premodulated therapy principles.
Explanation of the patented Target feature for precise electrode placement.
Description of the Sweep feature for wider area coverage in IFC treatments.
Details on Russian and Biphasic stimulation modes and parameters.
General information on High Volt therapy waveform and settings.
General information on Microcurrent therapy principles and settings.
General information on Ultrasound therapy principles and soundheads.
Contraindications, warnings, and precautions for electrical stimulation therapies.
Contraindications, warnings, and precautions specific to microcurrent treatment.
Contraindications, warnings, and precautions specific to ultrasound treatment.
General cautions for electrotherapy, including current density and patient susceptibility.
Guidelines for using and maintaining carbon and self-adhesive electrodes.
Information on lead wire care, replacement, and testing.
Explanation of current limit features and warning indicators.
Specific cautions for using microcurrent therapy.
Specific cautions for using ultrasound therapy.
Compliance with relevant IEC and FDA regulations for ultrasound equipment.
Diagrams showing the spatial distribution of the radiated field for soundheads.
Instructions for entering parameters for older model soundheads.
Procedure for calibrating soundheads to ensure proper operation.
Troubleshooting steps for soundhead overheating or being too cold.
Information on resolving the 'HEAD' error for undetected soundheads.
Guidance on resolving general error messages displayed by the device.
Overview of the device's general specifications.
Technical specifications for stimulation modalities.
Technical specifications for microcurrent therapy.
Technical specifications for High Volt therapy.
Technical specifications for Ultrasound therapy (850plus only).
Operating and storage environmental requirements for the device.
Instructions for cleaning and maintaining the device and its accessories.
Recommended maintenance schedule for the device, including technician tasks.
Information on routine calibration inspections for the Dynatron 850plus.
Explanation of device symbols and equipment classification.
Information on FDA compliant accessories and medical device reporting.
How the processor interacts with device components like keyboard, displays, and oscillators.
Methods for troubleshooting leads, pads, and basic device issues.
Information on installing software updates and replacement parts.
Checklist for final quality control of the Dynatron 950/850 Plus Stim.
| Electrodes | Self-adhesive, reusable |
|---|---|
| Type | Combination Therapy Device |
| Therapy Type | Ultrasound and Electrotherapy |
| Frequency | 1 MHz, 3 MHz |
| Power Output | Up to 100 mA |
| Modes | Continuous and Pulsed |
| Modulation | Frequency and amplitude modulation |
| Waveform | Symmetrical Biphasic |
| Channels | 2 independent channels |
| Timer | 1 to 60 minutes |
| Display | LCD Display |
| Power Supply | 100-240 V, 50/60 Hz |
| Design | Portable |