Regulatory Requirement
Venue 50 complies with regulatory requirements of the following European
Directive 93/42/EEC concerning medical devices.
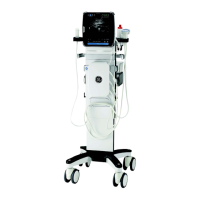
This manual is a reference for the Venue 50. It applies to all versions of the R4.x.x
software for the Venue 50 ultrasound system.
GE
P.O. Box 414, Milwaukee, Wisconsin 53201 U.S.A.
(Asia, Pacific, Latin America, North America)
GE Ultraschall Deutschland GmbH & Co. KG
Beethovenstrasse 239
Postfach 11 05 60
D-42655 Solingen GERMANY
TEL: 49 212.28.02.208; FAX: 49 212.28.02.431
 Loading...
Loading...