67
Appendix A Micro Dist Methods
A.1 Methods List
See Table 3 for descriptions of the Micro Dist methods. See Table 4 for a list of related
QuikChem methods.
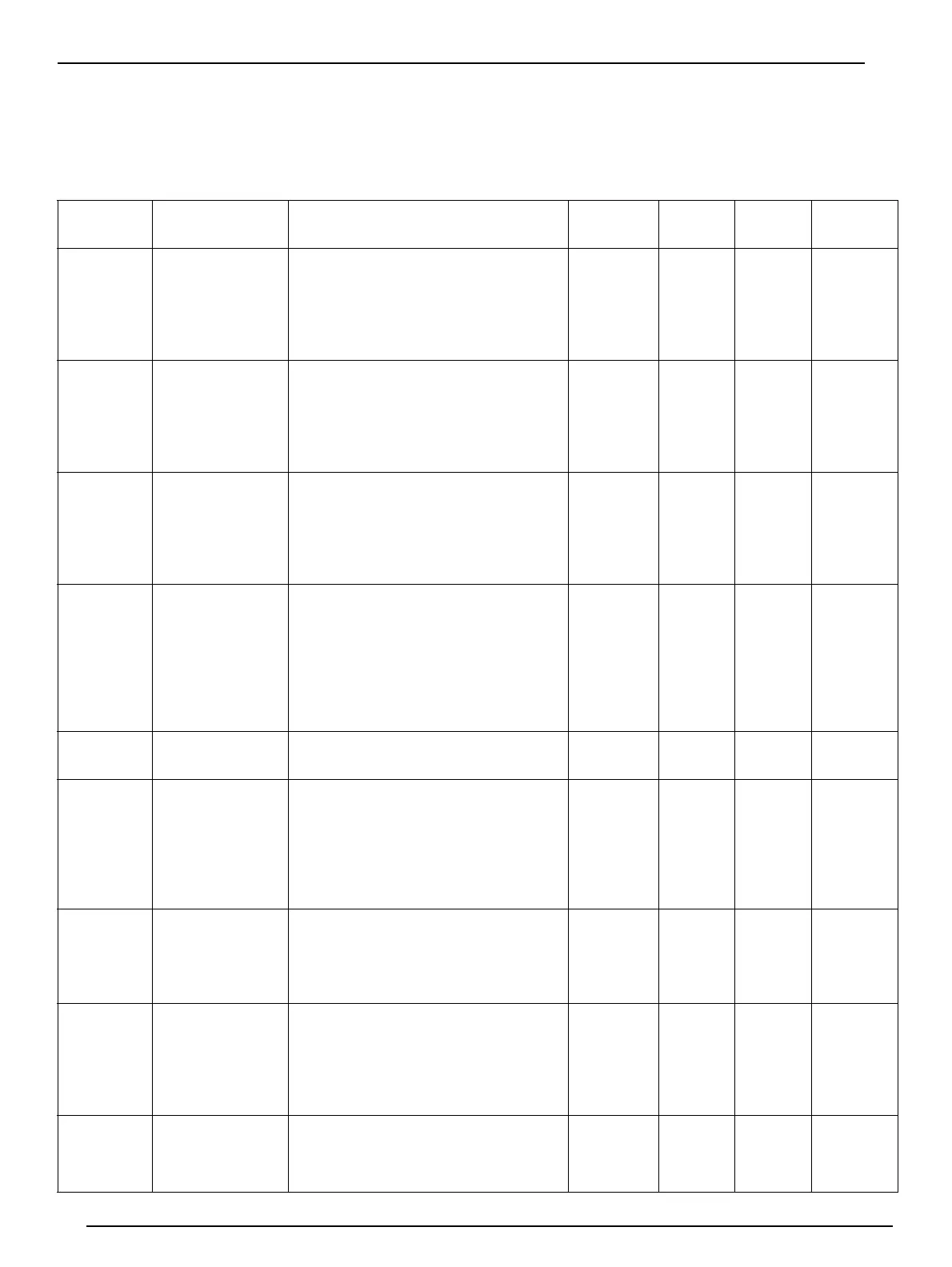
Table 3 Micro Dist Methods Summary
Micro Dist
Method No.
Matrix and
Chemistry
Collector Tube Trapping Solution
Recipe
Assembled
Pkg/21
User-Fill
Pkg/10
User-Fill
Pkg/50
User-Fill
Pkg/100
Cyanide–1
waters, solids,
strong acid
dissociable (SAD),
total cyanide
Add 1.5 mL or 1.55 g of 1.00 M
standardized NaOH solution to each tube.
When diluted to 6.0 mL, this gives 0.25 M
NaOH. Other standardized concentrations
can be used by adjusting the amount
added to each tube.
A17001 A17017 A17517 A17117
Cyanide–2
caustic extracts,
SAD, total cyanide
Add 1.5 mL or 1.55 g of 1.00 M
standardized NaOH solution. When
diluted to 6.0 mL, this gives 0.25 M
NaOH. Other standardized concentrations
can be used by adjusting the amount
added to each tube.
A17001 A17017 A17517 A17117
Cyanide–3
waters, solids,
weak acid
dissociable (WAD)
Add 1.5 mL or 1.55 g of 0.1.00 M
standardized NaOH solution. When
diluted to 6.0 mL, this gives 0.25 M
NaOH. Other standardized concentrations
can be used by adjusting the amount
added to each tube.
A17001 A17017 A17517 A17117
Cyanide–5
waters, solids,
SAD (total cyanide)
in the presence
of sulfide
Dissolve 0.80 g PbCO3 in 1 L of 1.00 M
standardized NaOH solution. Then, add
1.5 mL or 1.55 g of this solution to each
tube. When diluted to 6.0 mL, this gives
0.25 M NaOH and 0.8 mM Pb. Other
standardized NaOH concentrations can
be used by adjusting the amount added to
each tube.
A17011 A17017 A17517 A17117
Phenolics–
1
waters, solids,
4–AATP
No trapping solution. A17002 A17017 A17517 A17117
Sulfide–1
Acid
Soluble
Sulfides
waters, iodometric
determination
Prepare a 0.043 M zinc acetate solution
by dissolving the following: 8.78 g zinc
acetate dihydrate, 0.10 g conc. HCl, 880.0
g deionized water, and 43.2 g of a 37%
formaldehyde solution. Mix well until
dissolved.Add 2.0 mL or 2.0 g of the zinc
acetate solution to each tube.
A17003 A17017 A17517 A17117
Sulfide–2
waters/MTB
colorimetric
determination
Add 1.5 mL or 1.55 g of 1.00 M
standardized NaOH solution. When
diluted to 6.0 mL, this gives 0.25 M NaOH.
Other standardized concentrations can be
used by adjusting the amount.
A17001 A17017 A17517 A17117
Ammonia–1
waters, phenate
colorimetric or
ISE determination
Add 1.0 mL or 1.0 g of standardized
0.016 M sulfuric acid solution. When
diluted to 6.0 mL, this gives 0.003 M
sulfuric acid. Other standardized
concentrations can be used by adjusting
the amount.
Not
Available
A17017A A17517A A17117A
Ammonia–2
waters, solids,
nesslerization
Prepare a 0.13 M boric acid solution by
dissolving 8.0 g boric acid in 995.0 g
deionized water. Add 2.0 mL or 2.0 g of
this boric acid solution to each tube.
Not
Available
A17017A A17517A A17117A
 Loading...
Loading...