SPECIFICATIONS
Model 7600/7800 Operation Manual
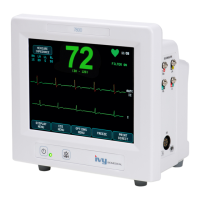
Medical Equipment
With respect to electric-shock, fire and
mechanical hazards only in accordance with
UL60601-1, CAN/CSA C22.2 No. 601.1, IEC 60601-2-27: 2005 (Second Edition); IEC 60601-1-4: (First Ed.) + Am.1: 1999 (Consolidated 1.1 Ed.)
ANSI/AAMI ES60601-1(2005), CAN/CSA C22.2 No. 60601-1(2008), IEC 60601-2-27 (2011), IEC 60601-1-8 (2006)
ANSI/AAMI ES60601-1 (2005) + AMD 1 (2012), CAN/CSA C22.2 No. 60601-1(2014),
IEC 60601-2-27 (2011), IEC 60601-1-6:2010 (Third Edition) + A1:2013, IEC 60601-1- 8: 2006 (Second Edition) + Am.1: 2012
Ivy Biomedical Systems, Inc. has declared that this product conforms with the European Council Directive 93/42/EEC Medical Device Directive
when it’s used in accordance with the instructions provided in the Operation and Service Manuals.
Eurasian Conformity (EAC): This product passed all conformity assessment (approval) procedures that correspond to the requirements of
applicable technical regulations of the Customs Union.
 Loading...
Loading...