10
9123D-eIFU-0917
CAUTION: FEDERAL LAW (U.S.A.) RESTRICTS THIS DEVICE TO SALE BY OR ON THE ORDER OF A PHYSICIAN.
For professional use. See instructions for use for full prescribing information, including indications, contraindications,
warnings, precautions and adverse events.
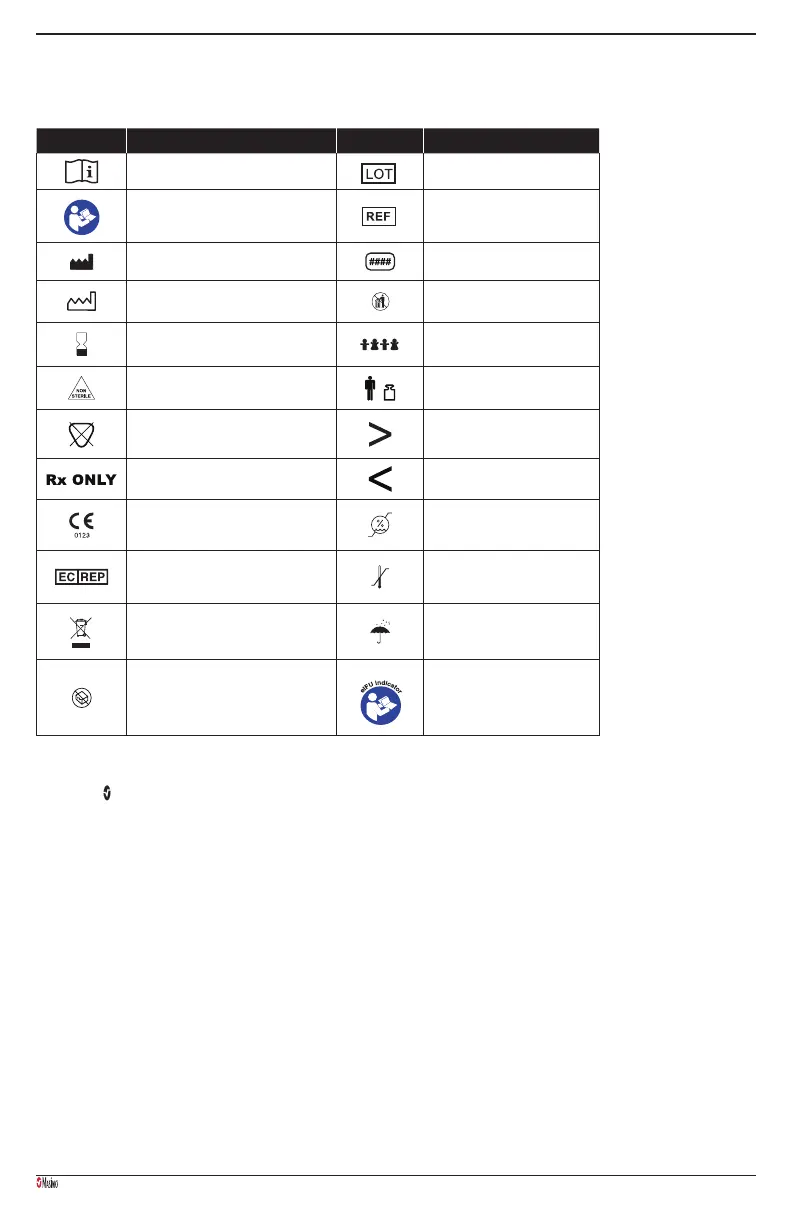
The following symbols may appear on the product or product labeling:
SYMBOL DEFINITION SYMBOL DEFINITION
Consult instructions for use Lot code
Follow instructions for use Catalogue number (model number)
Title: Graphic, Manufacturer Symbol, Masimo
PCX-1986
Revision A
DRO-23934
05/09
Manufacturer Masimo reference number
Date of Manufacture YYYY-MM-DD Do not discard
Use by YYYY-MM-DD Pediatric patient
Non-sterile Body weight
Not made with natural rubber latex Greater than
Federal law (USA) restricts this device to sale by or on
the order of a physician
Less than
Mark of Conformity to European Medical Device
Directive 93/42/EEC
Storage humidity limitation
Authorized representative in the European community Storage temperature range
Separate collection for electrical and electronic
equipment (WEEE).
Keep dry
Do not use if package is damaged
Instructions/Directions for Use/Manuals are
available in electronic format @
http://www.Masimo.com/TechDocs
Note: eIFU is not available for CE mark
countries.
Patents: referenced at www.masimo.com/patents.htm. Other patents pending.
M-LNCS and X-Cal are trademarks of Masimo Corporation.
Masimo, SET,
, CleanShield, LNCS and LNOP are federally registered trademarks of Masimo Corporation.
 Loading...
Loading...