21
AQUALAB VSA
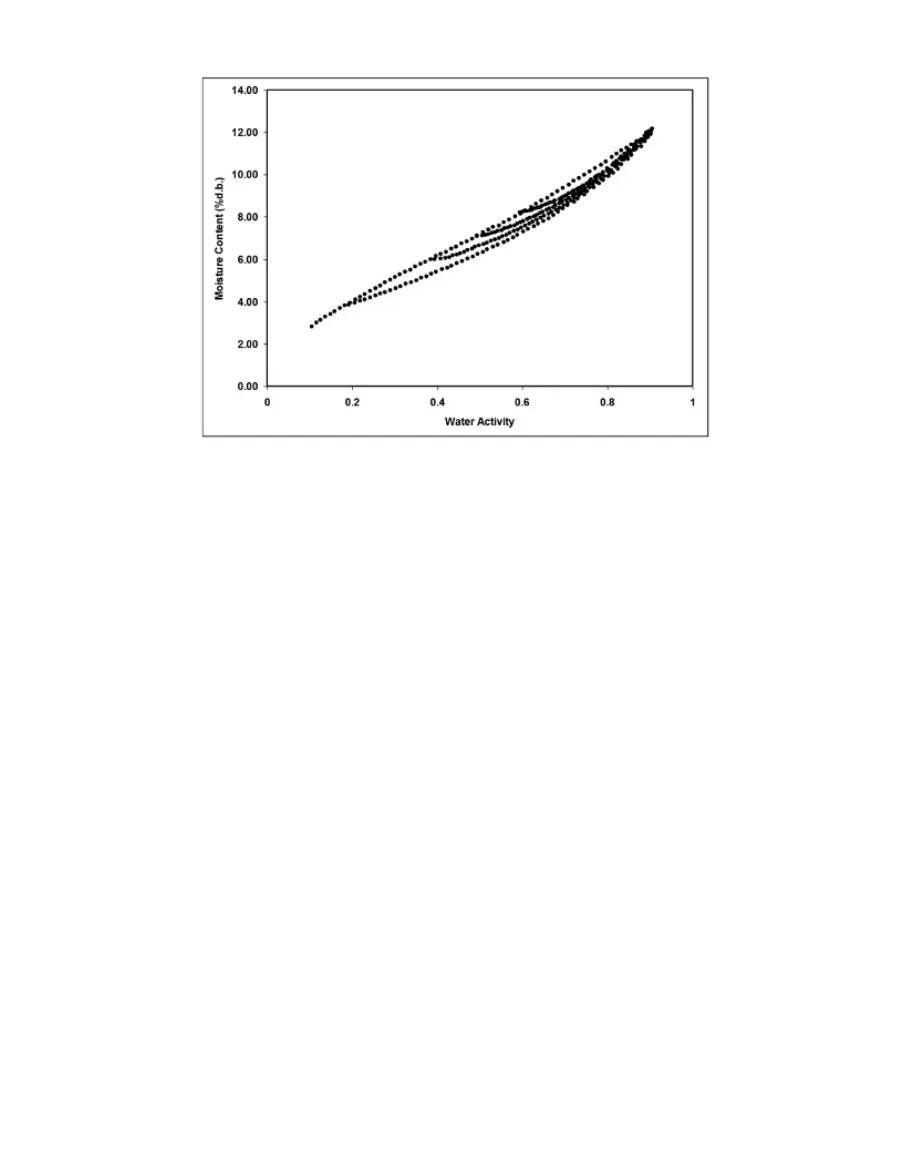
Figure 21 Scanning adsorption Curves from drying to varying a
w
These observations help clarify the point that an isotherm is not a single valued function. The
water content for any given water activity value depends on the wetting and drying history of
the sample.
It is possible to obtain isotherm data which appears to show hysteresis by failing to allow a
sample to equilibrate at each step, or by inducing changes in the water binding properties
of the matrix by wetting or drying. Here these cases are treated separately, and the term
"hysteresis" is reserved for situations where equilibrium is reached, but water contents of
wetted and dried samples still differ because of their history.
There are four primary models for hysteresis. These theories emphasize; capillary
condensation of porous solids, phase changes of nonporous solids, structural changes
within a solid matrix, and supersaturation of some solutes during desorption. Depending on
the composition of the sample, these theories explain why the water content of a desorption
process is greater than that for a wetting process.
1. The “ink bottle” model illustrates the capillary condensation of porous solids theory, in
which pores and capillaries fill and empty differently. Such a pore fills when the water
activity corresponding to the energy state of the larger radius is exceeded, but empties
only when the water activity drops below the energy state of the narrow neck radius.
2. A phase change of nonporous solid is illustrated by the fact that desorption from
rubbery state can reach equilibrium faster due to increased molecular mobility, while
adsorption into a glassy material can be slow due to restrictions in molecular mobility.
 Loading...
Loading...