1
Congratulations!
Thank you for buying a Milli-Q
®
water purication system.
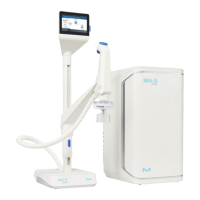
Milli-Q
®
IQ 7000 produces ultrapure water from a puried water source. Installation of this
product should be performed by a qualied service representative with access to qualied
installation documentation.
This user manual is a guide for use during the normal operation and maintenance of a
Milli-Q IQ 7000 water purication system. It is highly recommended to fully read this manual
and comprehend its contents before handling the water purication system.
System identication
System Catalog number Voltage Frequency
Milli-Q
®
IQ 7000 ZIQ7000T0 100-240 V 50-60 Hz
Manufacturing site:
Millipore SAS, 67120 Molsheim, France
For more information on your Milli-Q system, please call your local representative or visit our
website www.emdmillipore.com (North America) or www.merckmillipore.com (Rest of the World).
Intended use
The Milli-Q IQ 7000 is intended to produce ultrapure water from a puried water source
primarily for use in research and quality control in a variety of laboratories worldwide.
The product is designed to produce ultrapure water with specic characteristics (refer to the
requirements and specications section) when it leaves the water purication system, provided that
it is fed with water quality within specications and properly maintained as required by the supplier.
Merck KGaA does not warrant the product for any specic application. It is up to the user to
determine if the quality of the water produced by the product matches their expectations, ts
with norms/legal requirements and to bear responsibility resulting from the usage of the water.
The product is not intended to produce: water for injection, water for dialysis, sterile water for
irrigation or injection, bacteriostatic water for injection, sterile puried water in containers, and
sterile water for injection in container or ingestion. The product is not intended to be used in
explosive environments according to ATEX Directive – equipment & protective systems intended
for use in potentially explosive atmospheres. In addition the product is not intended as a Medical
Device, including In-Vitro Devices.
INTRODUCTION
 Loading...
Loading...