description of the problem, the frequency of the same problems, the quantity of sold equipment and your solution to the
problem (if any).
1.2.6. Additional Information
Please do not hesitate to contact MIR if you require additional information.
Manufacturer’s address:
MIR srl
Medical International Research
Via del Maggiolino, 125
00155 Rome
Italy
Tel.: +39/06/2275 4777
Fax.: +39/06/2275 4785
Email: mir@spirometry.com
1.2.7. Installation
Warning:
Before using Spirolab check internal battery charge level.
• turn the spirolab on, press then release the button on
the front of the unit.
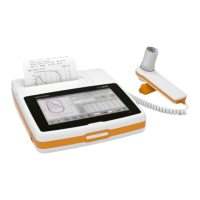
1.3. GENERAL
Spirolab is an “open circuit system” for the measurement of inspiratory and expiratory lung function parameters and
oximetry values, such as pulse rate and SpO2. It is suitable for basic lung function analysis of the mechanical respiratory
tract parameters. Three different respiratory tests can be performed:
• the forced vital capacity test (FVC),
• the slow Vital Capacity test (VC/IVC)
• the Maximum Voluntary Ventilation test (MVV)
Spirolab has been designed and manufactured to ensure the highest level of safety and the unit fully complies with the
stringent international EN 60601-1 and EN 60601-1-2 standards.
1.3.1. Technical Data
1.3.1.1. Spirolab unit
Max. current inside the unit
400 mA (with LCD at the maximum lightness)
NiMH rechargeable 7.2V battery pack (6
batteries, 1.2V each), 4000 mAh
CE 0476 EC mark for Medical Devices
FDA
 Loading...
Loading...