User Manual TXLD0012
P/N T2-UM-E-ENG Rev 10 Novoxel.com Page | 63
Chapter 9: General Guidance and Manufacturer’s Declaration: Essential
Performance & Electromagnetic Emissions & Immunity
Essential Performance
Temperature range shall vary between 385 and 405ºC.
Electromagnetic Emissions & Immunity
The medical device requires specific precautions regarding EMC and needs to be installed and put into
service cord to the EMC information provided in this document. This declaration only applies to the
following Novoxel
®
device: Tixel2 TXLD0012.
This device is intended for use in the electromagnetic environment specified below. The operator of the
device should verify that the device is operated in such an environment.
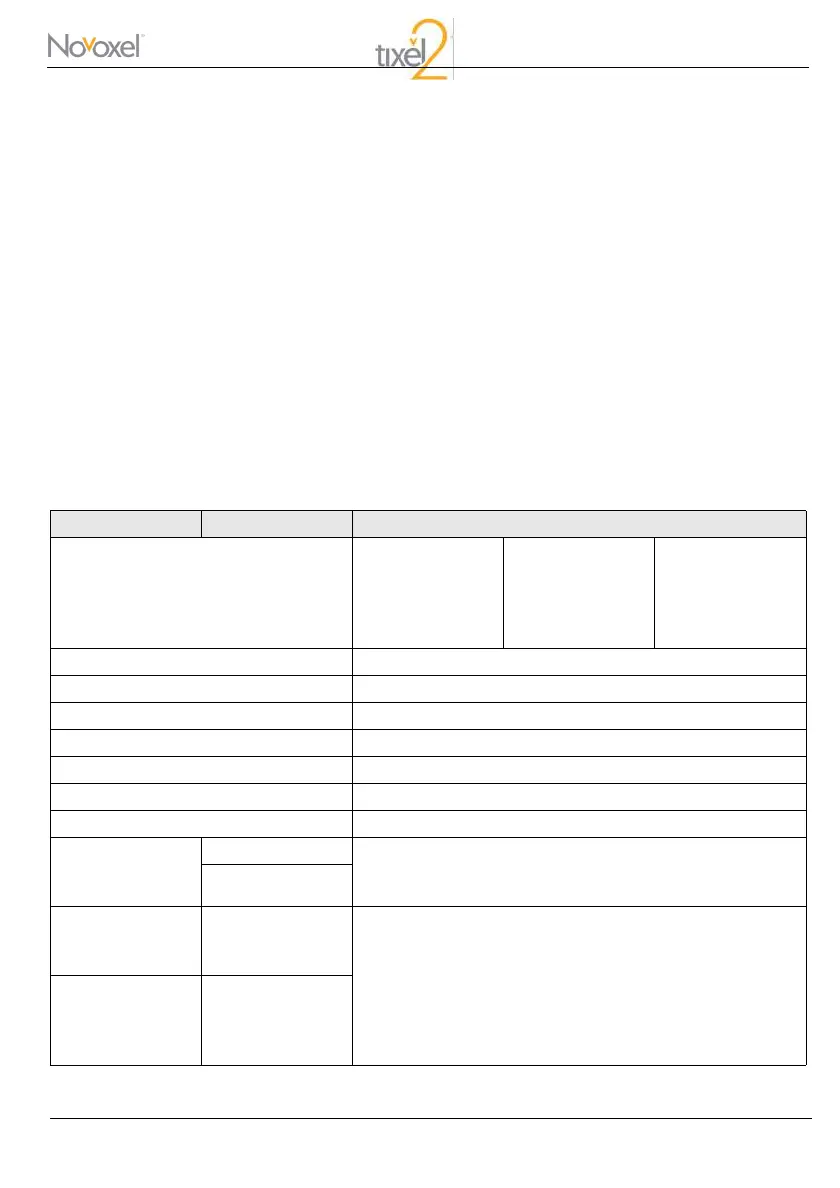
Emission Test Compliance Electromagnetic Environment - Guidance
Electrical Spec.
100-240 VAC ±
10%
T5A
50/60Hz
Up to 300W
Group 1 -
Energy Source Thermal
Tip Surface Material (Skin contact) Titanium
Console Weight 5400 gr
Handpiece Weight 330 gr
Total Device Weight 6100 gr
System Dimensions 26 L × 30 W × 31 H cm
Packaging Dimensions 57 L × 38 W × 44 H cm
RF emissions
CISPR 11
Group 1 The device uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to
cause any interference in nearby electronic equipment.
Class B
Harmonic
emissions
IEC 61000-3-2
Class B
The device is suitable for use in all establishments, including
domestic establishments and those directly connected to the
public low-voltage power supply network that supplies
buildings used for domestic purposes.
Vo l t a g e
fluctuations/flicker
emissions
IEC 61000-3-3
Complies
 Loading...
Loading...