11-2 Storage
11-1 Packaging, Sterilizing, and Drying
Store the product in a dry, clean location.
11. Sterilization
Follow local rules, regulations, and guidelines regarding the reprocessing of devices.
Remove the Protection Plug from the motor.
Insert the products into an FDA-cleared sterilization pouch that conforms to ISO 11607-1 and seal the pouch.
Perform steam sterilization with the following conditions.
Steam Sterilization Cycle
Type Gravity Displacement Pre-Vacuum (Dynamic Air Removal)
Temperature 132˚C 132˚C
Full Cycle Time 15 min. 4 min.
Drying Time 30 min. 30 min.
* The recommended sterilization procedures require the use of FDA-cleared sterilizers, sterilization trays, sterilization
wraps, biological indicators, chemical indicators, and other sterilization accessories labeled for the sterilization
cycle recommended
1)
2)
3)
22
CAUTION
Only Items Specified below can be autoclaved.
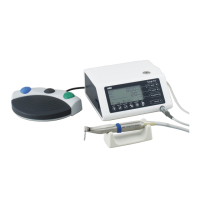
• Handpiece • Micromotor with Motor Cord • Handpiece Stand
• Internal Spray Nozzle • Tube Holder • Nozzle Holder
• Protection Plug • Calibration Bur • Cleaning Wire
CAUTION
• Use an FDA-cleared steam sterilizer to perform sterilization.
• Do not touch the product immediately after steam sterilization as it will be very hot and must remain in
a sterile condition.
• Do not perform steam sterilization on the product with other instruments even when it is in a pouch.
This is to prevent possible discoloration and damage to the product from chemical residue on other
instruments.
• Do not heat or cool the product too quickly. Rapid change in temperature could cause damage to the
product.
• Be sure to use sterilizers that can perform sterilization up to 135˚C. In some sterilizers, the chamber
temperature may exceed 135˚C. Do not use these sterilizers as failure of the product could occur.
Contact the sterilizer manufacturer for detailed information about cycle temperatures.
• Do not disconnect the motor cord from the micromotor.
• Steam sterilization is recommended for the product. The validity of other sterilization methods (such as
plasma sterilization or EOG sterilization) is not confirmed.
CAUTION
• After the sterilization and drying cycles are complete, remove the product immediately from the
sterilizer to store it.
• Store the product in a well ventilated place out of direct sunlight and within the range of temperature,
humidity and pressure specified in "1. Safety precautions prior to use".
• Sterilization is not guaranteed after the sterilization retention period specified by the manufacturer and
seller of the sterilization pouch has elapsed. If the sterilization retention period has elapsed, perform
sterilization again with a new sterilization pouch.
 Loading...
Loading...