5
On the packaging and on the identification plate of the instrument you may encounter the
following symbols, shown here with their meaning:
Caution, consult accompanying documents. Refer to safety-related notes in the
instructions for use accompanying this product.
Temperature limitation (Store at)
Use by
Manufacturer
Batch code/ Lot number
Catalog number
In vitro diagnostic medical device
This product fulfills the requirements of the European Directive 98/79/EC on in vitro
diagnostic medical devices.
Consult instructions for use
The system fulfills the Canadian and U.S. safety requirements (UL LISTED, in
accordance with UL 61010A-1:02 and CAN/CSA-C22.2 No. 61010-1-04).
LOT
IVD
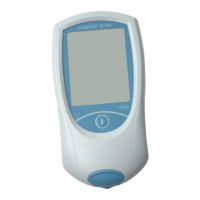
 Loading...
Loading...