Certification
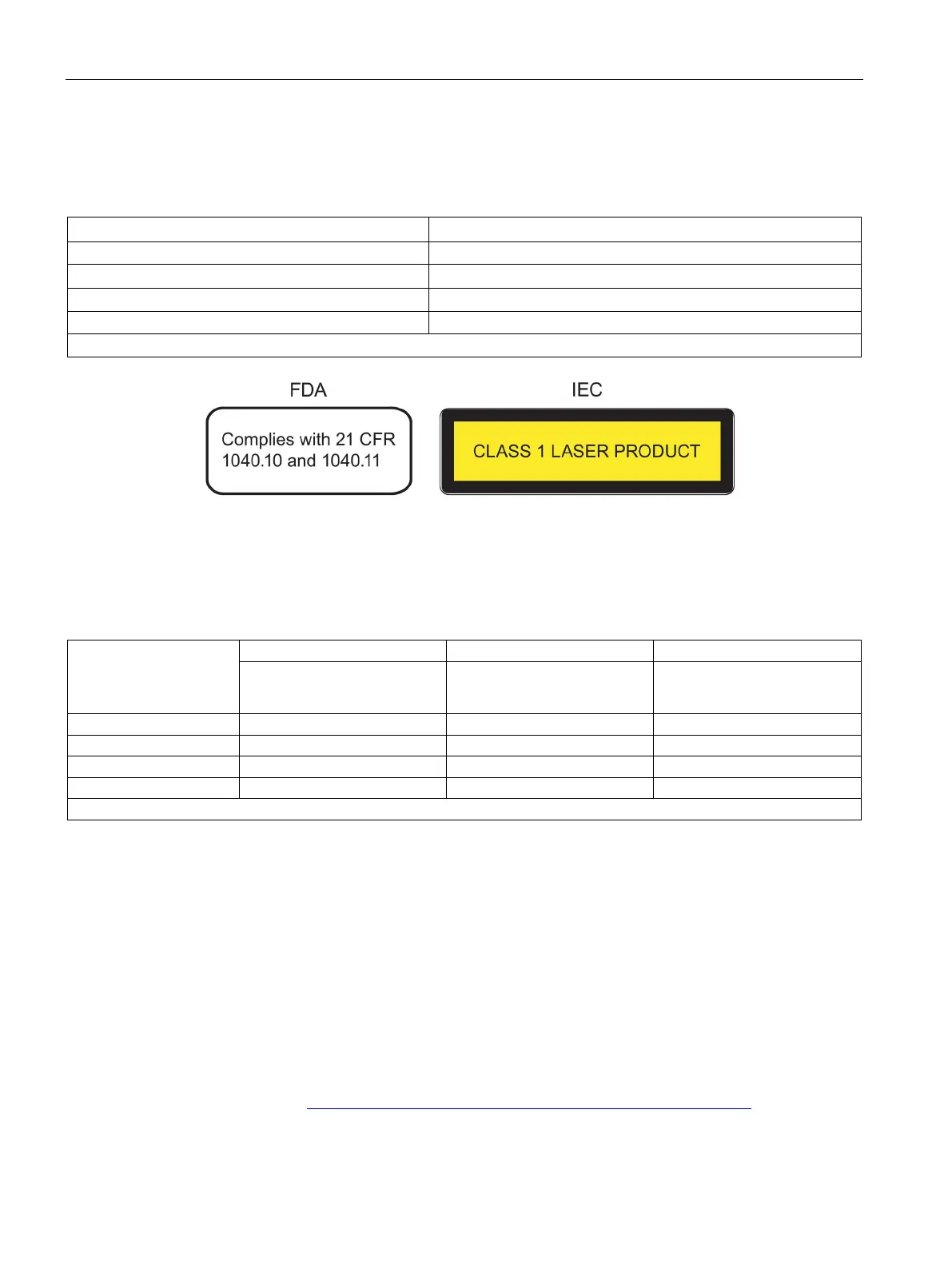
10.1 FDA and IEC marks
SCALANCE XR-500
108 Operating Instructions, 05/2017, A5E03275845-11
The following devices meet the FDA and IEC requirements listed below:
Fulfills FDA and IEC requirements
-
Note: With modular devices , the marking is on the MM900 media modules and the SFP and SFP+ transceivers.
Figure 10-1 FDA and IEC approvals
Mechanical stability (in operation)
IEC 60068-2-6 vibration *
5 - 9 Hz: 3.5 mm
9 - 150 Hz: 1 g
10 - 58 Hz: 0.075 mm
85 - 150 Hz: 1 g
15 g, 11 ms duration
6 shocks per axis
" When rack mounted with four securing points
The devices meet the requirements if you adhere to the installation and safety instructions
contained in this documentation and in the following documentation when installing and
operating the devices.
● "Industrial Ethernet / PROFINET Industrial Ethernet" System Manual
● "Industrial Ethernet / PROFINET - Passive network components" System Manual
You will find information on the system manuals in the section "Introduction (Page 5)", in
"Further documentation".
● "EMC Installation Guidelines" configuration manual
60612658 (http://support.automation.siemens.com/WW/view/en/60612658)

 Loading...
Loading...











