Waltron User Manual 101-016-B.3
3041 Silica Analyzer
Absorbance for liquids is defined as the negative logarithm of the transmittance:
A = - log
10
T = log
10
1/T = log
10
I
0
/I
1
I
0
= light intensity through the sample before colorimetric reaction
I
1
= light intensity through the sample after colorimetric reaction
In most cases the absorbance has a linear correlation with the sample concentration; for a linear
calibration line just the blank and span values (i.e. concentration with zero analyte and the
maximum expected concentration) are needed. Multiple analyses at each point are averaged to
provide a more accurate calibration line.
The absorbance ranges from 0 to 1, but it can go higher.
An absorbance of 0 at some wavelength means that no light of that particular wavelength
has been absorbed. The intensities of the sample and reference beam are both the same, so
the ratio I
0
/I
1
is 1. Log
10
of 1 is zero.
An absorbance of 1 occurs when 90% of the light at that wavelength has been absorbed
which means that the intensity is 10% of the blank value. In that case, I
0
/ I
1
is 100/10 (=10)
and log
10
of 10 is 1.
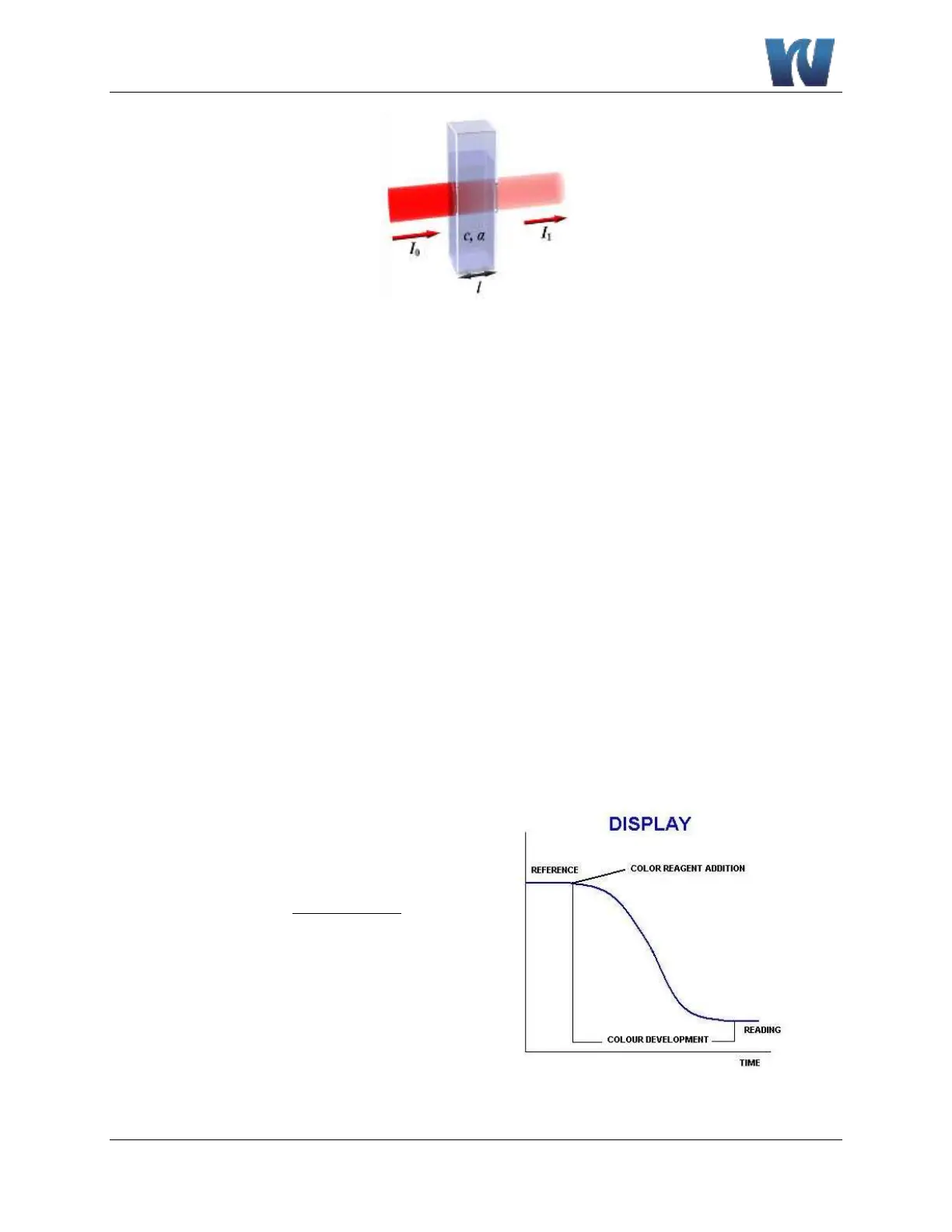
Absorption photometry (Colorimetry):
The method used is based on the formation of a colored complex of the analyte with a reagent.
Light with a specific wavelength is passed through the sample and reagent solution. . The
absorbance of this light by the reaction complex, measured by a photometer, is related to the
concentration of the analyte.
readingsensor
reference
Absorbance log
Figure 2.3: Color development.
 Loading...
Loading...