17
The Advanced
®
Model 3250/4250 Service Manual
Regulatory Description
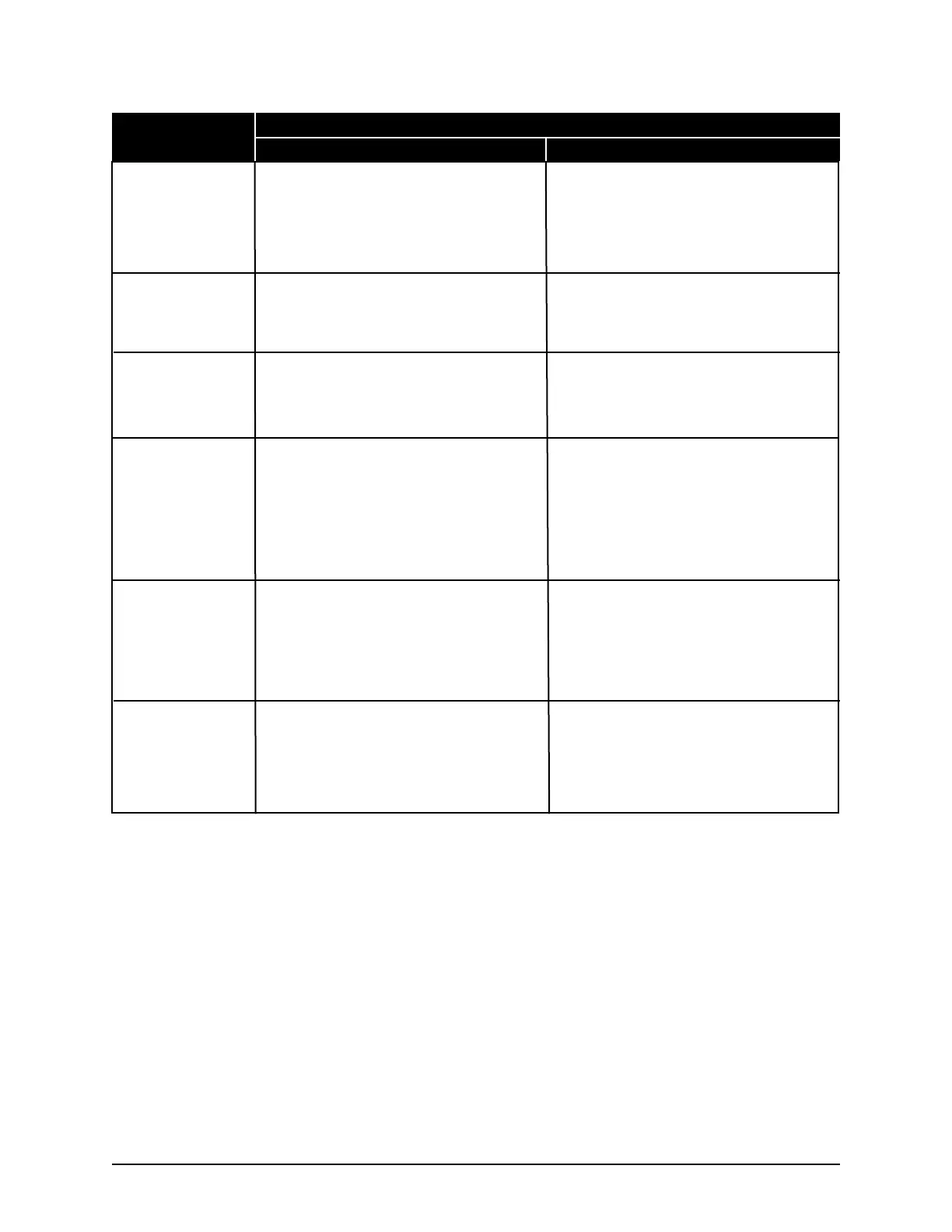
Approval Type Applies to Serial Suffix A - C Applies to Serial Suffix D and Higher
FCC - Part 15
Subpart B, Class B
Canadian ICES-003
Japan VCCI
U.S. FDA Listing
(3250 only)
Health Canada
License
(3250 only)
CE Declaration of
Conformity - RoHS
This device complies with Part 15 of the FCC
Rules. Operation is subject to the following two
conditions: (1) this device may not cause harmful
interference, and (2) this device must accept any
interference received, including interference that
may cause undesired operation.
This Class B digital apparatus complies with
Canadian ICES-003
Cet appareil numérique de la classe B est con-
forme à la norme NMB-003 du Canada.
-------------------------------
The osmometer, along with the calibrators and
controls manufactured by Advanced Instruments,
are listed with a U.S. Department of Health and
Human Services, Food and Drug Administration,
as:
Osmometer Class 1
Calibrators Class 2
Controls Class 1
The osmometer, along with the calibrators and
controls manufactured by Advanced Instruments,
are licensed with Health Canada, Therapeutic
Products Directorate, Medical Devices Bureau, as:
Osmometer Class 2
Calibrators Class 2
Controls Class 2
This product meets the intent of Directive
2002/95/EC for the Restriction of Use of Certain
Hazardous Substances in Electrical and Electronic
Equipment” as an exempt medical device per
Article 2, Paragraph 1 and per Annex 1, Category
8 and/or 9 of Directive 2002/96/EC.
This device complies with Part 15 of the FCC
Rules. Operation is subject to the following two
conditions: (1) this device may not cause harmful
interference, and (2) this device must accept any
interference received, including interference that
may cause undesired operation.
This Class B digital apparatus complies with
Canadian ICES-003
Cet appareil numérique de la classe B est con-
forme à la norme NMB-003 du Canada.
This Class B digital apparatus complies with
VCCI technical requirement V-3.
A CB report and certificate have been issued for
this product. A copy is available upon request.
The osmometer, along with the calibrators and
controls manufactured by Advanced Instruments,
are listed with a U.S. Department of Health and
Human Services, Food and Drug Administration,
as:
Osmometer Class 1
Calibrators Class 2
Controls Class 1
The osmometer, along with the calibrators and
controls manufactured by Advanced Instruments,
are licensed with Health Canada, Therapeutic
Products Directorate, Medical Devices Bureau, as:
Osmometer Class 2
Calibrators Class 2
Controls Class 2
This product meets the intent of Directive
2011/65/EU for the Restriction of Use of Certain
Hazardous Substances in Electrical and Electronic
Equipment”.
 Loading...
Loading...