84
2321 E EN 20130722
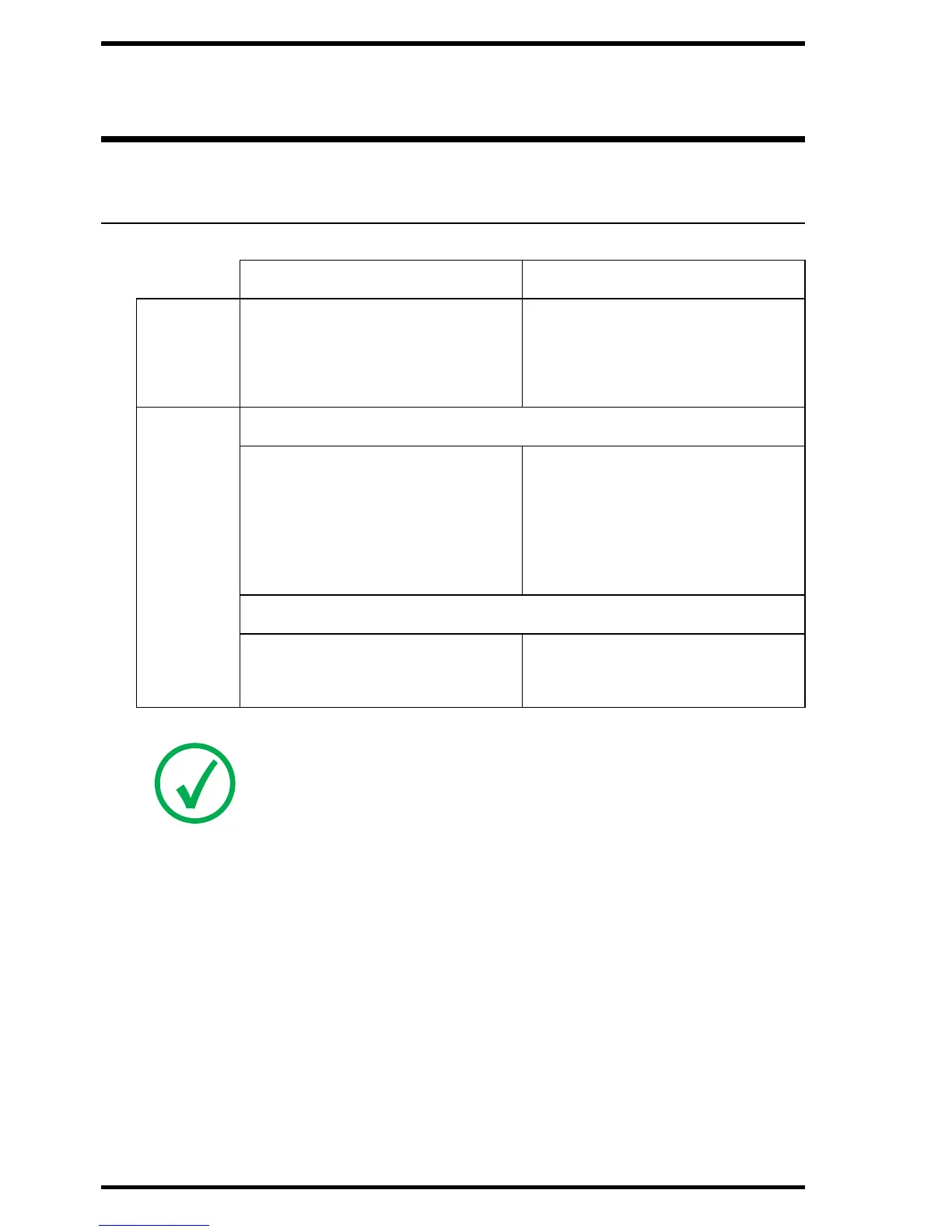
DX-G/DX-M
Compliance
Certificates
Europe USA/Canada
EMC EN 60601-1-2:2007
EN 300 330-2 V1.1.1:2001
EN 301 489-1 V1.3.1:2001
FCC part 15
CSA 22.2 No. 601.1.2
Safety Medical Equipment
IEC 60601-1:1988 +A1: 1991:
+ A2:1995
IEC 60601-1: 2005
UL60601-1:2003
CSA C 22.2No.601.1:1990 +
S1:1994 + A2:1998
ES 60601-1 1st Edition; CAN
CSA 22.2 no. 60601-1-08
Laser
IEC 60825-1:1993 + A1:1997
+ A2:2001
CFR parts 1040.10 and 1040.11
CSA-E60825-1-03
Note: The DX-G/DX-M is in compliance with the EG regulation 93/42/EEC
Directive (Medical Device).
 Loading...
Loading...