R5914634 /00 MDSC-852750
Indicates the device meets the requirements of the applicable EC directives/
regulations.
Indicates compliance with Part 15 of the FCC rules (Class A or Class B).
Indicates the device is approved according to the UL Recognition regulations.
MEDICAL – GENERAL MEDICAL EQUIPMENT
AS TO ELECTRICAL SHOCK, FIRE AND MECHANICAL HAZARDS ONLY
IN ACCORDANCE WITH ANSI/AAMI AS60601-1:2005/(R)2012, CSA CAN/CSA-C22.2
NO. 60601-1:14
Indicates the device is approved according to the UL regulations for Canada and US.
Indicates the device is approved according to the UL Demko regulations.
Indicates the device is approved according to the CCC regulations.
Indicates the device is approved according to the VCCI regulations.
Indicates the device is approved according to the KC regulations.
Indicates the device is approved according to the BSMI regulations.
Indicates the device is approved according to the PSE regulations.
Indicates the device is approved according to the RCM regulations.
Indicates the device is approved according to the EAC regulations.
Caution: Federal law (United Stated of America) restricts this device to sale by or on the
order of a licensed healthcare practitioner.
Important information
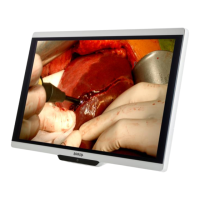
 Loading...
Loading...