Chapter 5: Quality and process control 51
Procedure Perform this procedure for the CD4 low count, CD4 normal count,
CD4 percentage, Hb low concentration, Hb normal concentration,
and Hb high concentration.
To prepare process controls:
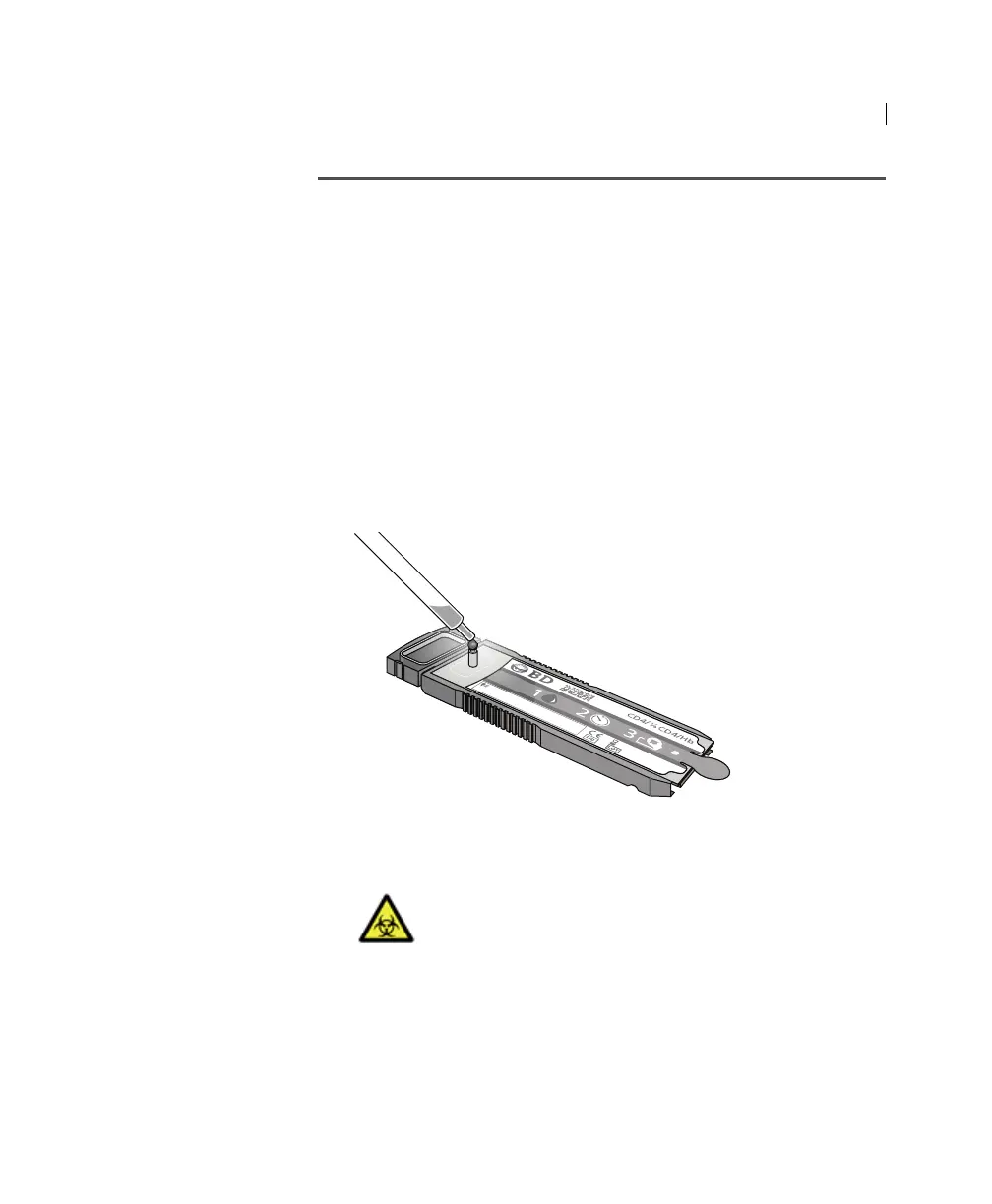
1. Carefully dispense the process control into the inlet port of the
cartridge. Hold the cartridge by its ridges only.
2. Make sure the process control reaches the top of the inlet port.
Make sure the cartridge is level, with the barcode side up, at all
times. Make sure that process control appears in the part of
the channel not covered by the channel protector, next to the
containment zone.
3. Close the cartridge cap securely. Make sure both latches on the
cap snap closed.
Caution: Biological! If necessary, use a cloth
dampened with bleach diluted to 0.5% sodium
hypochlorite concentration to clean excess process
control outside the containment zone. Be careful to
not contaminate the inlet port or introduce any
debris into the underside of the cartridge. Do not
smear, contaminate, or damage the barcode. If you
drop the cartridge into a contaminated area, discard
the cartridge into a biohazardous waste container
and start over with a new cartridge.
 Loading...
Loading...