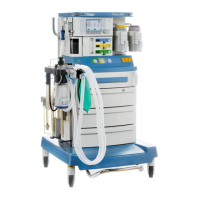
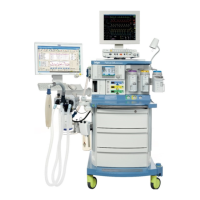
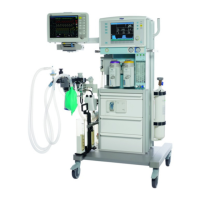
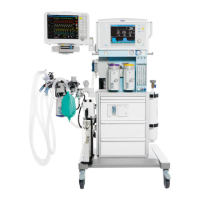
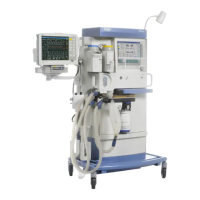
Known limitations of Dräger anesthesia devices regarding use in long-term
ventilation
WARNING: The following information is based on our currently available knowledge as of the
date of this letter. It only applies to Dräger anesthesia devices still being marketed. It is most
likely not complete or exhaustive. If you detect important points that are missing, please let us
know.
WARNING: Dräger as the manufacturer cannot and is not allowed to market or promote or sign-
off such off-label use of Dräger anesthesia devices. The following information is therefore
provided only to give the responsible health care professional a better basis for decision-making.
If a device is used off-label, the user does so under their own responsibility and at their own
(liability) risk.
1 General instructions
The following proposals support the targets that the off-label-use is as safe as possible, and that as many
anesthesia devices as possible will maintain their ventilation capacities, even under long-term ventilation.
Refer to the following website https://www.draeger.com/en-us_us/Home/novel-coronavirus-outbreak for
an additional check list that you may provide at every workplace. Please be aware, that this
customer letter is more detailed and prevails over the additional check list.
2 Prerequisites & preparation
2.1 Training of medical personnel
- Anesthesia devices have a different working principle and different user interface (e.g. different
operating modes) than intensive care ventilators. Therefore, medical personnel using the
device must be well trained and familiar with the unique performance characteristics of
the devices.

 Loading...
Loading...