190
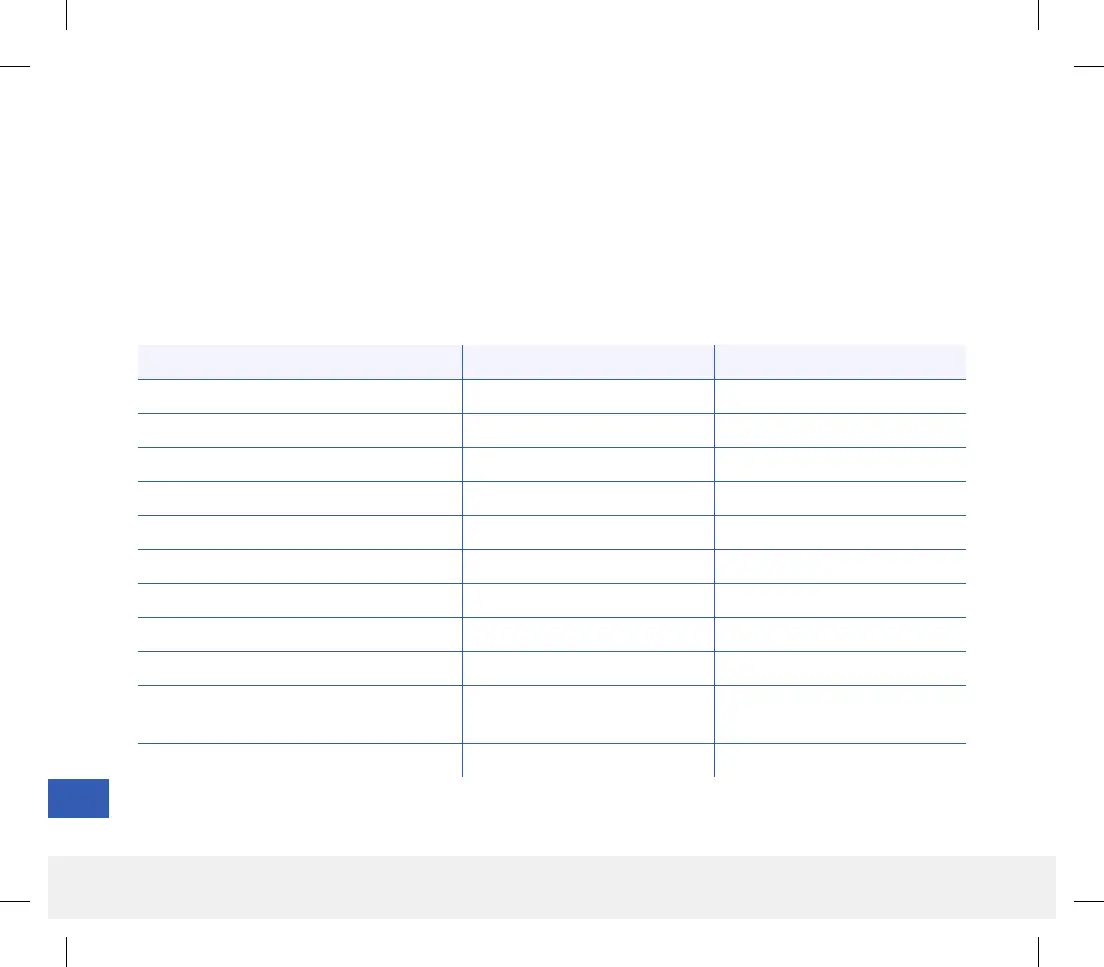
Event Type Number of Events Patients (n = 125)
Pain/Discomfort 6 5 (4.0%)
Redness/Erythema 2 1 (0.8%)
Dermatitis at Patch Location 2 1 (0.8%)
Bruising 2 1 (0.8%)
Skin Hyperpigmentation 2 1 (0.8%)
Paresthesia 1 1 (0.8%)
Syncope-vasovagal 1 1 (0.8%)
Pain 1 1 (0.8%)
Device Fragment not Recovered 2 2 (1.6%)
Additional Procedure to Remove Sensor
Following First Attempt
3 2 (1.6%)
TOTAL 22 12 (9.6%)
Table 18 – Adverse Events
Safety
Both the PRECISE II and PRECISION studies lasted 90 days, and the number of related adverse events was recorded.
The Eversense CGM System was well tolerated in the studies. During the studies’ 15,921 sensor wear days, there were
no unanticipated adverse events. Twenty-two adverse events were reported in 12 participants, including one serious
adverse event related to the removal procedure. There were no infections. Mild irritation, pain and redness at the
sensor insertion site were observed at a low rate of occurrence. None of the adverse events resulted in hospitalization.
19
LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 190LBL-1602-01-001 Rev N_Eversense User Guide_mgdL_R1.indd 190 2/26/20 12:59 PM2/26/20 12:59 PM
 Loading...
Loading...