7
0.1M FERROUS AMMONIUM SULFATE TITRANT CONCENTRATION
0.1M FERROUS AMMONIUM SULFATE TITRANT CONCENTRATION
METHOD ID: HI0010EN
DESCRIPTION
Method for the standardization (titer determination) of 0.1M
Ferrous Ammonium Sulfate (FAS) titrant solution against Potassium
Dichromate (K
2
Cr
2
O
7
). The results are expressed in M (mol/L).
REFERENCE
Standard Methods for the Examination of Water and Wastewater
21
st
Edition, Method 5220B
ELECTRODE
• HI3131B Combination ORP Electrode
REAGENTS
• HI70444 25% Sulfuric Acid
• HI70436 Deionized Water (1 gal)
• Ferrous Ammonium Sulfate (ACS Grade)
• Potassium Dichromate (ACS Grade)
ACCESSORIES
• HI70300L Storage Solution (500 mL)
• HI7071 Electrode Fill Solution (30 mL x 4)
• HI740036P 100 mL Plastic Beakers (10 pcs)
• Analytical Balance with 0.0001 g resolution
• 100 mL Class A Volumetric Flask
• 500 mL Class A Volumetric Flask
• 10 mL Class A Volumetric Pipette
TITRANT PREPARATION
• Carefully weigh 19.607 grams of ferrous ammonium sulfate.
• Carefully transfer the salt to a 500 mL Class A volumetric flask. Add
approximately 300 mL of deionized water, and mix to dissolve.
• Add 40.00 mL of 25% sulfuric acid (HI70444) to the flask. Invert
the solution to mix.
• Allow the flask to return to room temperature.
• Bring the flask to volume with deionized water, mix well.
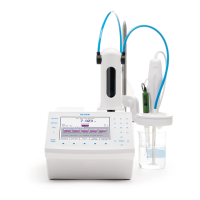
DEVICE PREPARATION
• Connect the ORP electrode to the titrator.
• Install a 25 mL burette filled with 0.1M ferrous ammonium
sulfate on pump one and verify that no air bubbles are present
in the burette or tubing. If necessary prime the burette until all
the air has been removed completely.
• Press from the main screen. Use the arrow keys to
highlight HI0010EN 0.1M FAS and press .
ELECTRODE PREPARATION
• Prepare the ORP electrode according to the procedure in the
manual.
SAMPLE PREPARATION
• Crush approximately 2 grams of potassium dichromate and dry it
for 2 hours at 150°C. Cool to room temperature in a desiccator.
• Carefully weigh approximately 0.49 grams of dried potassium
dichromate.
• Record the exact weight of the sample once the balance has
stabilized with an accuracy of 0.0001 grams.
• Carefully transfer the salt to a 100 mL Class A volumetric flask.
Add approximately 80 mL of deionized water, and mix to
dissolve. Once the salt is completely dissolved bring the flask to
volume with deionized water, mix well.
• Use a Class A volumetric pipette to transfer exactly 10.00 mL of
the solution to a clean 100 mL plastic beaker.
• Add 25.00 mL of 25% sulfuric acid (HI70444) to the beaker.
• Add deionized water to the 50 mL mark on the beaker.
ANALYSIS
• Place the beaker under the stirrer assembly and lower it to
immerse the electrode and stirrer. Ensure that the reference
junction of the ORP electrode is 5 to 6 mm below the surface. If
necessary add extra deionized water.
Note: The dispensing tip should be slightly submerged in the
sample.
• Press . You will be prompted to enter the weight of the
analyte (weight of potassium dichromate). Use the numeric
keypad to enter the exact weight and press to start the
analysis.
• At the end of the titration, after detection of the equivalence
point, “Titration Completed” will appear with the result. The
result is expressed in M (mol/L) of ferrous ammonium
sulfate.
• Remove the ORP electrode and stirrer from the sample and rinse
them thoroughly with deionized water.
• Record the result.
Note: For improved accuracy, repeat this procedure a minimum
of three times and calculate the average value.
For methods utilizing 0.1M ferrous ammonium sulfate titrant
solution, follow the steps below to enter the titer/standardized
value.
• Select the method utilizing 0.1M ferrous ammonium sulfate
• Press from the main screen.
• Using the arrow keys, highlight Titrant Conc. and press
.
• Use the numeric keypad to enter the standardized (titer) value
of the titrant then press .
• Press to exit the View/Modify Method screen and
select Save Method and press .
 Loading...
Loading...