TITRATION OF BASES
Weak bases with pK
b
’s up to about 11, which do not ionize with water, can be titrated in non-aqueous solvents. These bases include
aliphatic and aromatic amines, basic nitrogen heterocycles, alkali metal and amine salts of acids, and many other organic basic
compounds. Titrating a weak base with a strong acid titrant requires a basic solvent that is as weak as possible. Water and alcohols
allow the titration of medium strength bases such as aliphatic amines (pK
b
= 4 to 5), but not the titration of weaker bases such
as pyridine (pK
b
= 8.8). Glacial acetic acid works well for weak bases and has been used extensively. Less basic solvents such as
acetone, acetonitrile, and nitromethane extend the range of titrable compounds.
The endpoint for non-aqueous titrations are usually determined potentiometrically using a pH glass electrode, a modified calomel or
double junction reference electrode with a low-flow rate reference junction. Good potentiometric titration curves are obtained in most
solvents, except those with very low dielectric constants such as benzene, chloroform and others, when high electrical resistance of the
solvent causes unstable potentials.
2.2.6. PRECIPITATION TITRATIONS
Precipitation titrations allow for faster analysis compared to the old gravimetric analysis, where a precipitate is formed, filtered, dried
and weighed to analyze a compound. Typically silver halides, silver thiocyanate and a few mercury, lead, and zinc salts are titrated
using this method. The chemical reactions must form an insoluble salt and precipitate out quickly in order to be analyzed by this
method. When the reaction is not quick, a back titration can be used. A measured excess of the precipitating reagent (titrant) is
added to force the reaction to occur, and then unreacted titrant is then titrated with a standard solution of another reagent.
2.2.7. REDOX TITRATIONS
There are a number of oxidation-reduction reactions that can be used to determine unknown concentration by titration. If the reaction
goes to completion, is fast and has an analytical signal available to follow it, a titration can be performed. The term “fast” means
that each addition of titrant is reacted completely and the sensing electrode is able to detect the change in solution in less than one
second.
Redox titrations are potentiometric titrations where the mV signal from a combination ORP (redox) electrode (usually with a platinum
indicator electrode) is used to follow the reaction of oxidant/reductant. The electrode potential is determined by the Nernst equation
and is controlled by the oxidant reductant ratio.
Visual indicators such as Ferrion are also available. The oxidized and reduced form of the indicator will have different colors and can
be used to determine the end point.
Various reductants can be determined by titrants with oxidants such as potassium permanganate, potassium chromate or iodine.
Commonly used reductants that are used as titrants include sodium thiosulfate, and ferrous ammonium sulfate.
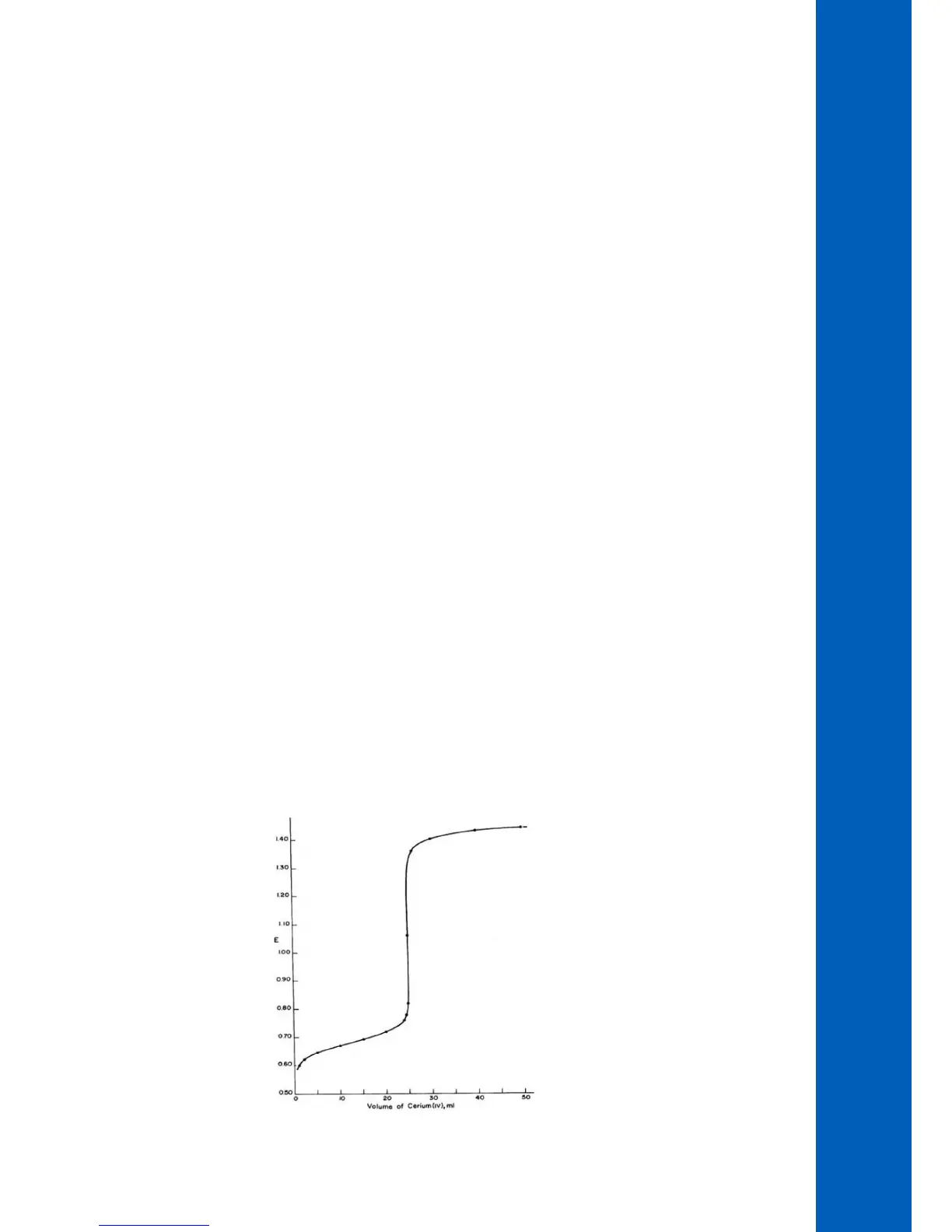
As with Acid-Base titrations the potential changes dramatically at the equivalence point.
Figure 8
 Loading...
Loading...