An indicator electrode that responds to the metal ion can be used to monitor the titration progress. The titration curve will appear
similar to a usual potentiometric titration. Complexation indicators change color at the endpoint as all metal ions are “consumed”, or
complexed, by the titrant. The titration curve will appear similar to a potentiometric titration when using an indicator electrode that
responds to the metal ion (see Figure 6).
Figure 6
2.2.4. ION SELECTIVE TITRATIONS
The most popular ion selective titration is an acid-base titration. The hydrogen ion concentration is specifically measured and
monitored during the titration process to locate the equivalence point. Using an ion selective electrode (ISE) as the indicator
electrode, the potentiometric signal (in mV) is used to directly follow a specific ion’s concentration (or activity).
Examples of ISE titrations include titrating fluoride with an aluminum titrant using a fluoride ISE, chloride with silver nitrate using a chloride
ISE, sodium with a sodium ISE, etc. The equivalence point can be determined by plotting the mV value vs. the amount of titrant added.
2.2.5. NON-AQUEOUS SOLVENT ACID-BASE TITRATIONS
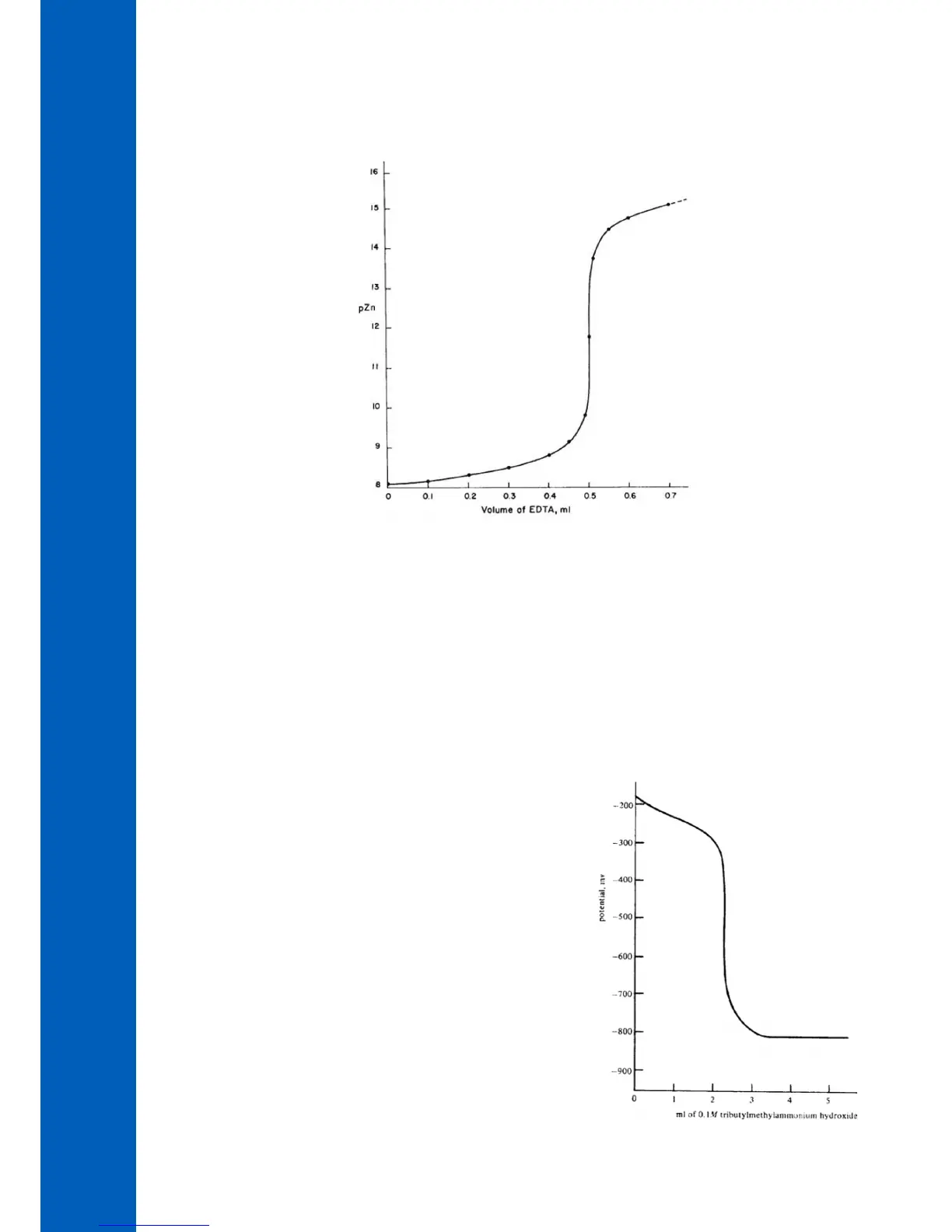
Non-aqueous solvents must be used to titrate very weak acids and bases due to Figure 7
the inherent leveling effect water has on all acids and based dissolved in it.
A wide variety of weak acids and bases can be titrated using non-aqueous
solvents. Mixtures of acids or bases can often be individually analyzed in a single
sequential titration.
TITRATION OF ACIDS
Weak acids with pK
a
’s up to about 11 can be titrated in non-aqueous solvents.
These include carboxylic acids, enols, phenols, imides, sulfonic acids, and
inorganic acids. Water or lower alcohols are suitable for titrating medium to strong
acids (pK
a
less than 5). Titrating a weaker acid with a strong base titrant requires
a solvent less acidic than water or ethanol/methanol. Solvents such as acetone,
acetonitrile, t-butyl alcohol, dimethylformamide, isopropanol and pyridine have
been found to work well for acid-base titrations of strong, medium and weak
acids/bases. Titrants include alcoholic potassium hydroxide and various sodium or
potassium alkoxides in a 10:1 mixture of benzene/methanol. The best titrants are
quaternary ammonium hydroxides (such as tetrabutylammonium hydroxide) due
to good solubility of tetraalkylammonium salts of the titrated acids and the clean
potentiometric titration curve obtained (see Figure 7).
 Loading...
Loading...