2. TYPES OF TITRATIONS
2.1. TITRATIONS ACCORDING TO THE MEASUREMENT METHOD
2.1.1. AMPEROMETRIC TITRATIONS
An amperometric titration is performed by placing two electrodes (often a metal ISE and a reference electrode) into the sample
solution and holding the potential of the metal electrode at a selected voltage. The current that flows, due to the oxidation or
reduction of a reactant or product, is plotted vs. volume of titrant to provide the titration curve and locate the equivalence point.
Changes in the current are due to changes in the concentration of a particular species (being oxidized or reduced at the electrode).
Generally the reaction between the analyte and titrant forms a new species. Depending on the titration, the reactants are electroactive
and the products are not, or vice-versa. Amperometric titration curves look like two straight lines intersecting at the equivalence point,
this is due to the change in the electroactivity of the solution.
Many metal ions can be amperometrically titrated using a precipitation, complexation or redox reaction. Some metal ions and species
that can be determined in this manner include silver, barium, halides, potassium, magnesium, palladium, molybdate, sulfate,
tungstate, zinc, bismuth, cadmium, fluoride, indium, thallium, iodine, and gold.
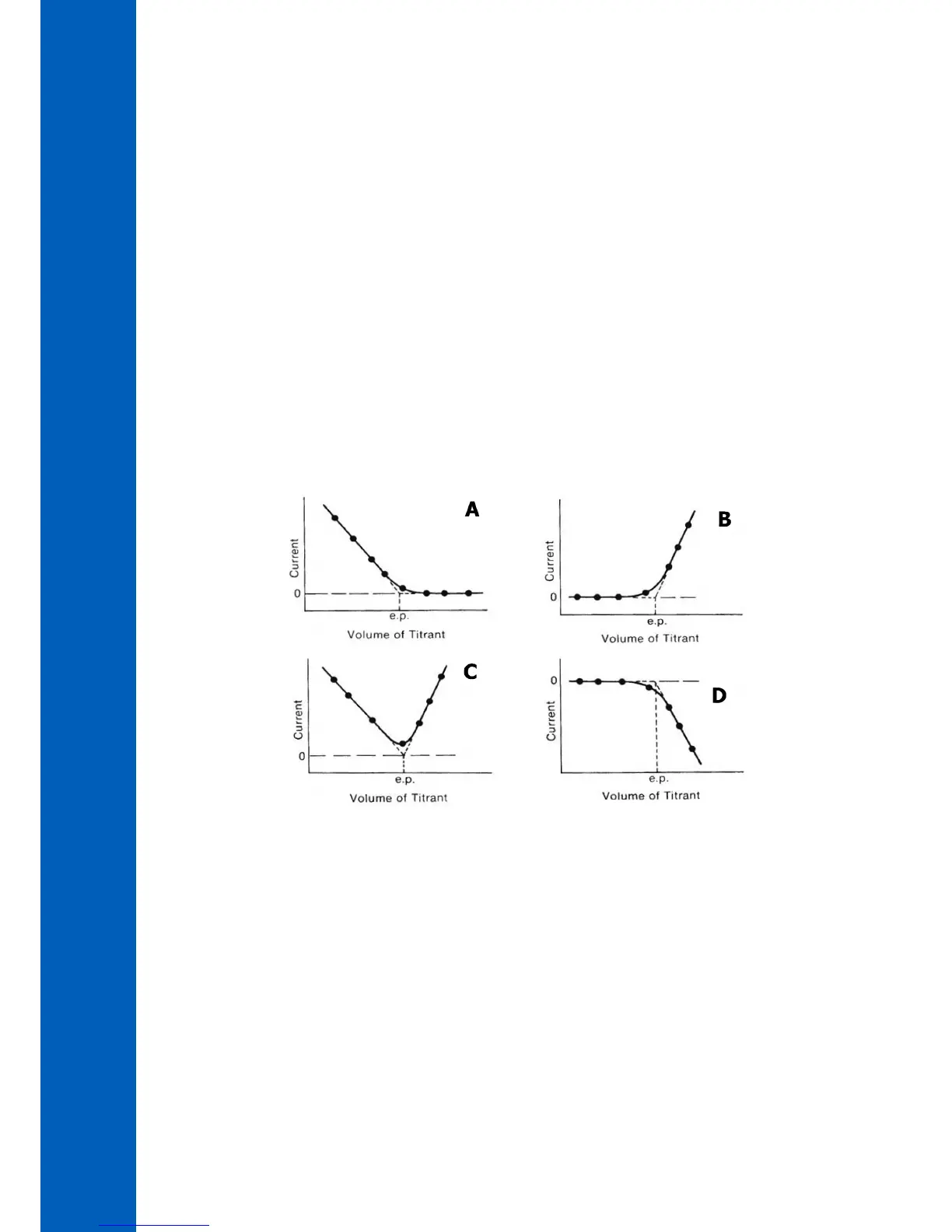
Figure 1 shows four amperometric titrations and their endpoints. In graph “A” the analyte is electroactive and gives current but the
reacted species does not. In “B” the reactant is not active but the titrant is. In “C” both the analyte and titrant are active and both
give current flow. Graph “D” shows the same situation as “B”; however, the current has an opposite sign (the titrant is reduced).
Figure 1
2.1.2. POTENTIOMETRIC TITRATIONS
Potentiometric titrations are done by measuring the voltage across the solution using an electrode system. An electrode system
consists of an indicator electrode and a reference electrode. As titrant is added the variations in the potential of the indicator
electrode, with respect to the reference electrode, are monitored to show the progress of the titration.
Potentiometry is the measurement of a potential under conditions of zero current flow. The measured potential can then be used
to determine the analytical quantity of interest, generally a component concentration of the analyte solution. The potential that
develops in the electrochemical cell is the result of the free energy change that would occur if the chemical phenomena were to
proceed until the equilibrium condition has been satisfied.
There are many types of titrations where potentiometry can be used,e.g., pH electrodes for acid-base titrations, platinum ORP electrodes in redox
titrations, ion selective electrodes, such as chloride or fluoride for a specific ion titration, and silver electrodes for argentometric (silver-
based) titrations.
An example of potetiometric titrations are shown below. Figure 2 “A” is the pH of a solution vs. the volume of titrant and “B” is the
potential from a chloride electrode vs. the volume of AgNO
3
.
 Loading...
Loading...