9
0.02M SILVER NITRATE TITRANT CONCENTRATION
0.02M SILVER NITRATE TITRANT CONCENTRATION
METHOD ID: HI0200EN
DESCRIPTION
Method for the standardization (titer determination) of 0.02M Silver
Nitrate (AgNO
3
) titrant solution against Sodium Chloride (NaCl). The
results are expressed in M (mol/L).
REFERENCE
AOAC Official Methods of Analysis, Official Method 941.18
ELECTRODE
• HI4115 Silver/Sulfide Combination ISE
REAGENTS
• HI70448 0.02M Silver Nitrate (1 L)
• HI70406 Sodium Chloride (20 g)
• HI70427 1.5M Nitric Acid Solution (500 mL)
• HI70436 Deionized Water (1 gal)
ACCESSORIES
• HI7072 Electrode Fill Solution (4 x 30 mL)
• Analytical Balance with 0.0001 g resolution
• 150 mL Glass Beaker
• 100 mL Class A Volumetric Flask
• 5 mL Class A Volumetric Pipette
DEVICE PREPARATION
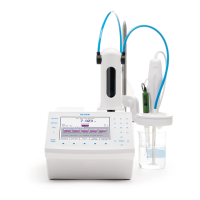
• Connect the Silver/Sulfide electrode to the titrator.
• Install a 25 mL burette filled with 0.02M silver nitrate (HI70448)
on pump one and verify that no air bubbles are present in the
burette or tubing. If necessary prime the burette until all the air
has been removed completely.
• Press from the main screen. Use the arrow keys to
highlight HI0200EN 0.02M Silver Nitrate and press .
ELECTRODE PREPARATION
• Prepare the Silver/Sulfide electrode according to the procedure in
the manual.
SAMPLE PREPARATION
• Crush approximately 2 grams of sodium chloride (HI70406)
and dry it for 2 hours at 140°C. Cool to room temperature in
a desiccator.
• Weigh 0.20 g of dried sodium chloride with an accuracy of
0.0001 g. Transfer the salt to a 100 mL volumetric flask.
Add approximately 80 mL of distilled water and mix. Dissolve
completely before bringing to volume.
• Use a Class A volumetric pipette to transfer exactly 5.00 mL of
prepared standard solution to a 150 mL glass beaker and add
distilled water to the 100 mL mark on the beaker.
• Add 10.00 mL of 1.5M nitric acid (HI70427) to the beaker.
ANALYSIS
• Place the beaker under the stirrer assembly and lower it to
immerse the Silver/Sulfide electrode and stirrer. Ensure that
the reference junction of the electrode is 5 to 6 mm below the
surface. If necessary add extra deionized water.
Note: The dispensing tip should be slightly submerged in the
sample.
• Press . You will be prompted to enter the weight of the
analyte (weight of sodium chloride). Use the numeric keypad
to enter the exact weight and press to start the analysis.
• At the end of the titration, after detection of the equivalence
point, “Titration Completed” will appear with the result. The
result is expressed in M (mol/L) of silver nitrate.
• Remove the electrode and stirrer from the sample and rinse them
thoroughly with deionized water.
• Record the result.
Note: For improved accuracy, repeat this procedure a minimum
of three times and calculate the average value.
For methods utilizing 0.02M silver nitrate titrant solution,
follow the steps below to enter the titer/standardized value.
• Select the method utilizing 0.02M silver nitrate.
• Press from the main screen.
• Using the arrow keys, highlight Titrant Conc. and press
.
• Use the numeric keypad to enter the standardized (titer) value
of the titrant then press .
• Press to exit the View/Modify Method screen. Use
the arrow keys to highlight Save Method and press .
 Loading...
Loading...