Calibration frequency: yearly.
Maintenance: cleaning using the IDRONAUT “Conductivity sensor cleaning
solution”.
1.15.5 The oxygen sensor (standard 150bar and 700 bar versions)
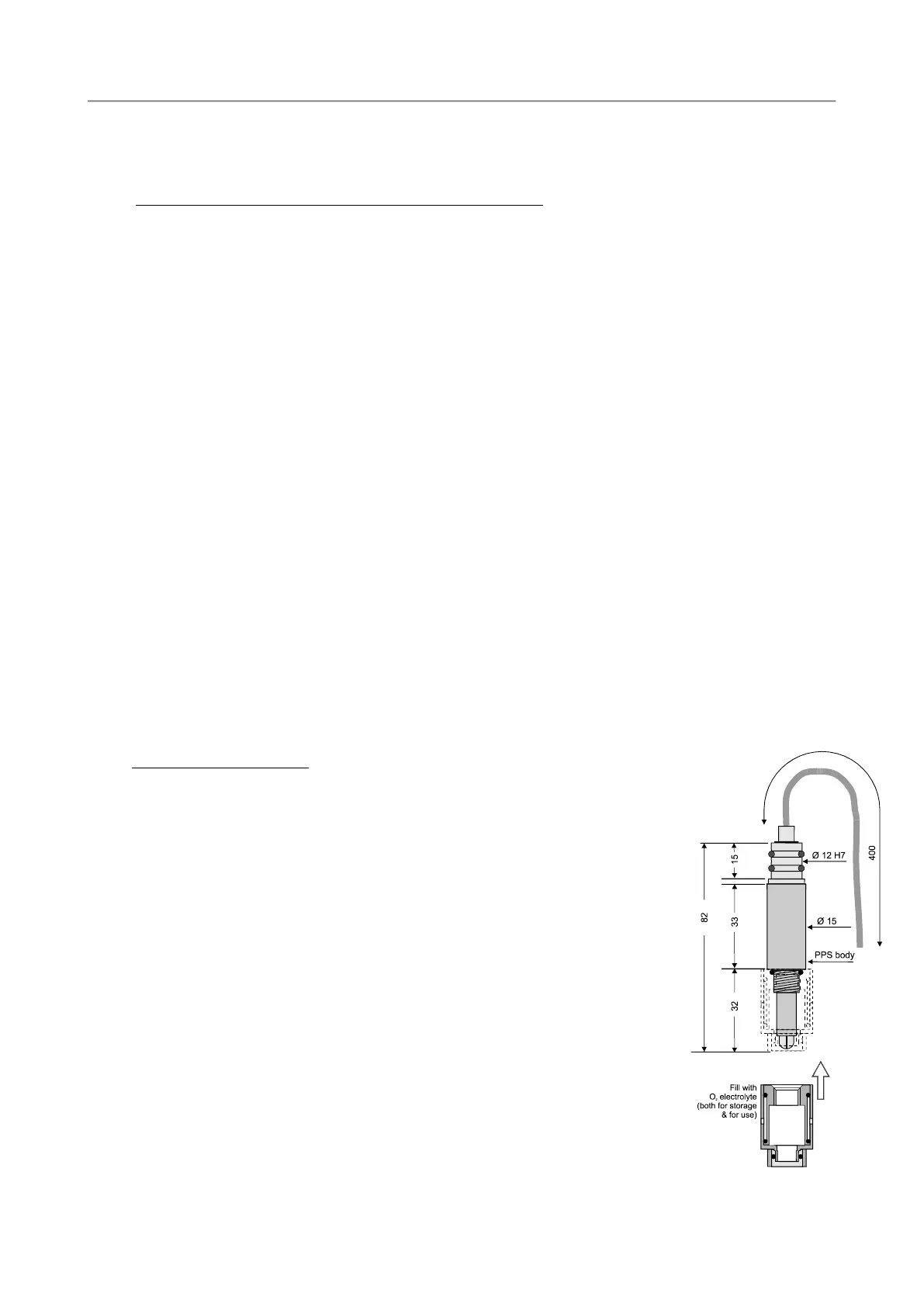
The oxygen sensor is of the polarographic type and consists of two half-cells, the anode and the cathode.
The anode is a silver tube inside the sensor, which encircles a glass body where a platinum wire, forming
the cathode, is sealed. The platinum wire (cathode) ends at the tip of the sensor where the glass body is
rounded. A special membrane cap with a gas-permeable replaceable membrane screws onto the sensor.
The inside of the cap is filled with a special electrolyte which allows the current (measuring) to flow
between the anode and the cathode. The membrane is shielded from accidental bumps by a protective
ring. The anode acts as a reference cell, providing a constant potential with respect to the cathode. The
cathode, where oxygen is consumed or reduced, is separated from the sample to be analyzed by a thin
layer of electrolyte and a special composite membrane. The electrolyte permits the chemical reaction to
occur whereas the membrane constitutes a barrier against ions and other substances. By applying a
polarizing voltage to the half-cells, the sensor develops a current proportional to the concentration of
oxygen in the sample in front of the cathode. Oxygen from the sample is drawn across the membrane,
at the sensor tip, in the area of the cathode. The applied polarization voltage is such that the sensor only
responds to oxygen. The sensor is insensitive to nitrogen, nitrous oxide, carbon dioxide and other gases.
In order to avoid stray ground current leaks, in case of membrane leaks, the anode is kept at ground
potential while the cathode is polarized at a fixed negative voltage. The oxygen sensor limits stirring
effects on the measurement and reads at least 97% of the true value, even with a stagnant aqueous
sample. This is because the very small cathode area and special cathode geometry, associated with a
unique composite membrane, minimize the consumption of the oxygen contained in the sample in
contact with the membrane. The function of this sensor depends on the reduction of oxygen at the
cathode, as expressed by the formula:
O2 + 2 H2O + 4e
-
>>> 4 OH
-
The developed electrons represent the measuring current and are supplied by the silver/silver chloride anode.
Standard version, 150 bar
Type: polarographic with Pt/Ir cathode and
Ag(99.99%) anode.
Measurement range: 0... 50 ppm 0… 500% sat.
Accuracy: 0.1 ppm 1 % sat.
Resolution: 0.01 ppm 0.1% sat.
Polarization voltage: 650 mV DC.
Response time: 3s (green membrane @20°C)
0.9 s (blue membrane @20°C)
Max Pressure: 150 bar.
Sensor body: plastic and titanium.
Compensation: automatic compensation of pressure and
thermal variations.
Life: 2 years if intensively used to perform
continuous monitoring, up to 4 years if
used weekly to perform daily profiling or
monitoring.
Calibration frequency: weekly.

 Loading...
Loading...