Instructions for use PROPHYflex 4
7 Reprocessing steps in accordance with ISO 17664 | 7.7 Sterilisation
28 / 34
7.7 Sterilisation
Sterilisation in a steam steriliser (autoclave) in
accordance with EN 13060 / ISO 17665-1
NOTICE
Contact corrosion due to moisture.
Damage to product.
▶ Immediately remove the product from the steam steriliser after the steril-
isation cycle.
Note
Prior to attaching the powder container, all powder-conducting parts and air
channels must be absolutely dry. Screw together the powder container and
handpiece only in the cold state.
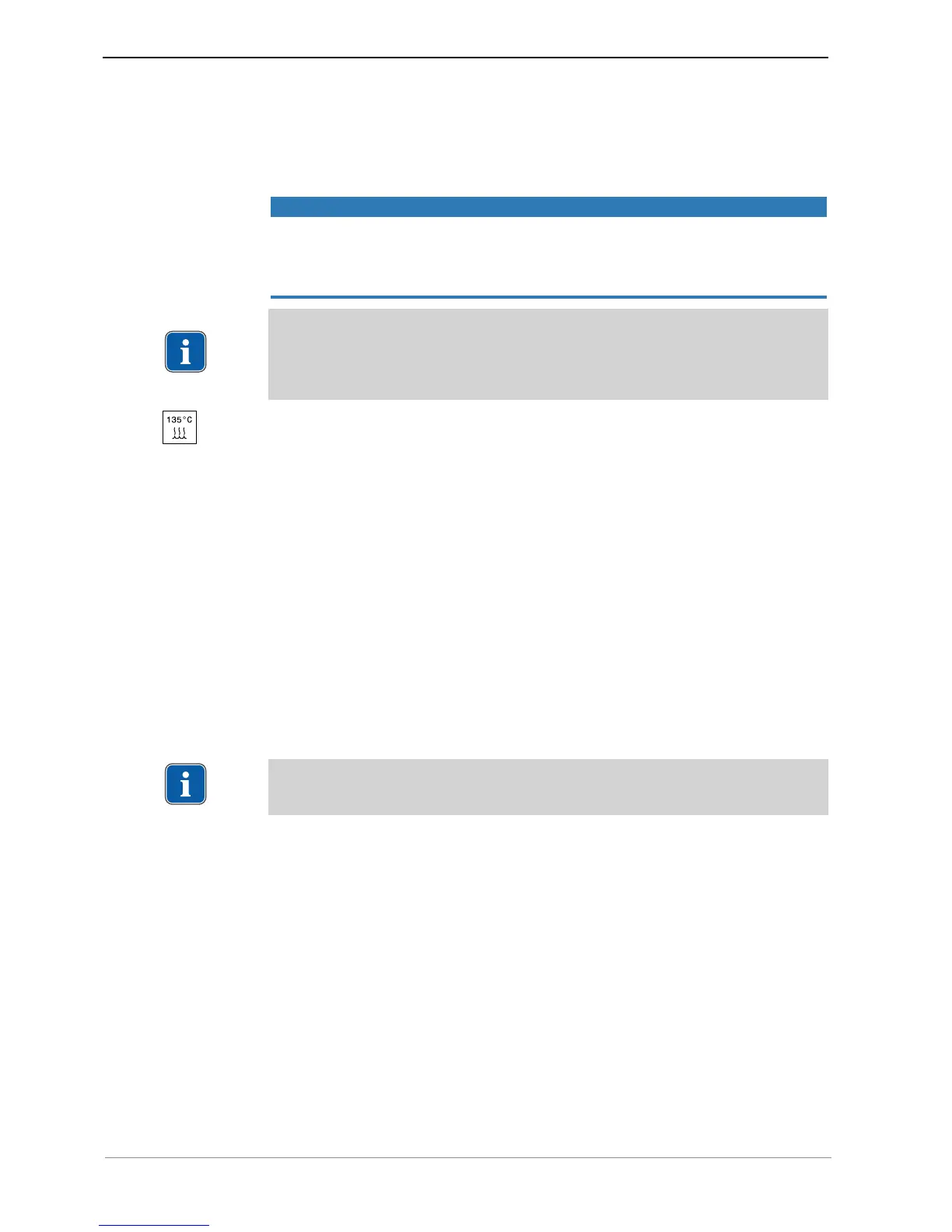
The KaVo medical device has a maximum temperature resistance up to 138 ℃
(280.4 °F).
Select a suitable procedure (depending on the available autoclave) from the fol-
lowing sterilisation processes:
▪ Steriliser with triple pre-vacuum:
- at least 3 minutes at 134 °C -1 °C / +4 °C (273 °F -1.6 °F / +7.4 °F)
▪ Steriliser using the gravity method:
- at least 10 minutes at 134 °C -1 °C / +4 °C (273 °F -1.6 °F / +7.4 °F) or
alternatively
- at least 60 minutes at 121 °C -1 °C / +4 °C (250 °F -1.6 °F / +7.4 °F)
▶ Use according to the manufacturer's Instructions for Use.
7.8 Storage
Reprocessed products must be stored appropriately such that they are protec-
ted from germs (as far as possible) and dust, in a dry, dark, cool room.
Note
Comply with the expiry date of the sterilised items.
 Loading...
Loading...