1.
Introduction
United States Federal Law restricts this device to sale by or on the order of a
licensed healthcare practitioner, licensed by the law of the jurisdiction in which
they practice, to use or order the use of this device.
Only qualified personnel should perform ultrasound scanning of human
subjects for medical diagnostics.
This ultrasound system meets the acoustic output emission guidelines established by the
U.S. Food and Drug Administration (FDA). Acoustic output quantities have been
measured, and are displayed, in accordance with the standards listed under Guidance
Documents.
Indications for Use
The ultrasound system is intended for use by a qualified physician for ultrasound evaluation
of Ophthalmic; Fetal; Abdominal (renal, GYN/Pelvic); Intra-operative (abdominal,
thoracic(cardiac), and vascular); Intra-operative (Neuro); Laparoscopic; Pediatric; Small
organ (thyroid, breast, testes, etc); Neonatal cephalic; Adult Cephalic/Transcranial; Trans-
rectal; Trans-vaginal; Trans-esoph.(non-Card); Musculoskeletal (Conventional);
Musculoskeletal (Superficial); Cardiac Adult; Cardiac Pediatric; Trans-esoph. (Cardiac);
Intra-cardiac; Peripheral vessel.
Note: The availability of application may be limited based on country or region of use.
Device Description
The ZS3 Ultrasound Platform and products derived there from (for example, but not limited
to, the ZS3 and the z.onepro with and without the SP UI option) is used for ultrasound
evaluation of the following applications: Laparoscopic, Fetal, Abdominal, Intra-operative,
Pediatric, Ophthalmic, Small Organ/Parts (breast/testes, thyroid, etc.), Transvaginal,
Transrectal, Transcranial, OB/GYN, Cardiac, Pelvic, Neonatal/Adult Cephalic, Vascular,
Tissue Elasticity, Contrast Imaging, Musculoskeletal, Superficial Musculoskeletal and
Peripheral Vascular applications. Users include ultrasound imaging technicians
(sonographers) and physicians. This ultrasound system may be used in a hospital (e.g.
imaging laboratory, emergency room, patient bedside, and operating room), medical clinic,
physician’s office or a mobile imaging center.
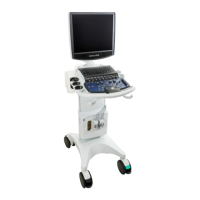
The ZS3 Ultrasound Platform consists of two major components: 1) Cart; and, 2)
Transducer(s). The Cart contains the software driven imaging electronics and user
interfaces (keyboard, monitor, handles, etc.). It houses the microprocessor, memory,
amplifiers and power supplies for the microprocessor. It sends electrical currents to and
receives electrical pulses from the compatible transducers. The Cart performs the
calculations involved in processing the data to produce the displayed ultrasound images.
 Loading...
Loading...