Optomed Aurora 41
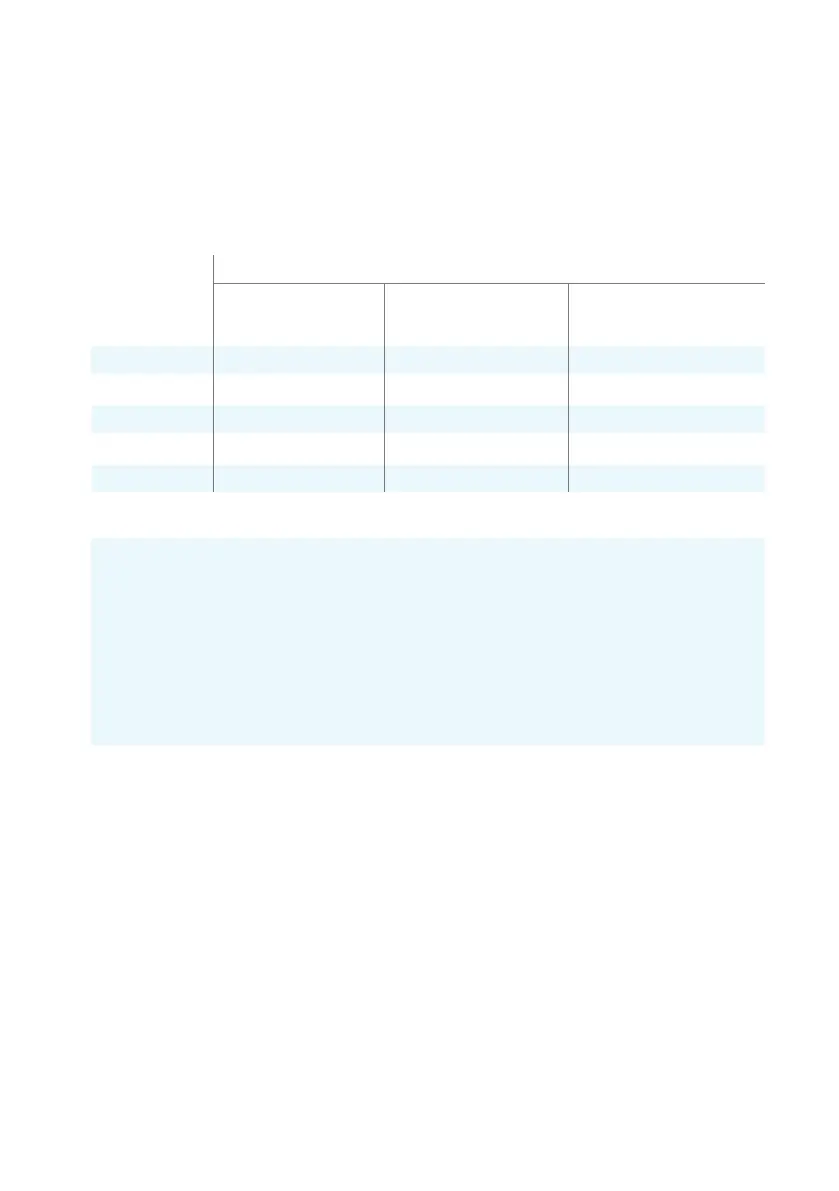
Rated maximum
output power
of transmitter
(W)
0.01
0.1
1
10
100
Separation distance according to frequency of transmitter (m)
from 150 kHz to 80 MHz
d = 1.2 √P
from 80 MHz to 800 MHz
d = 1.2 √P
from 800 MHz to 2.7 GHz
d = 2.3 √P
0.12 0.23
0.38 0.73
1.2 2.3
0.12
0.38
1.2
3.8
3.8
7.3
12
12 23
SEPARATION DISTANCES
Optomed Aurora maintains basic safety and performance when used in the electromagnetic environment
in which radiated RF disturbances are controlled. The customer or the user of Optomed Aurora can help
prevent electromagnetic interference by maintaining a minimum distance between portable and mobile
RF communications equipment (transmitters) and Optomed Aurora as recommended below, according to
the maximum output power of the communications equipment.
For transmitters rated at a maximum output power not listed above, the recommended separation
distance d in meters (m) can be estimated using the equation applicable to the frequency of the
transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to
the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is aected
by absorption and reflection from structures, objects and people.
Important information |
6.9 Compliance
The classification of Optomed Aurora according to the standard IEC 60601-1:2005+A1:2012
• Optomed Aurora is internally powered ME equipment
• Optomed Aurora has type BF applied parts
• Protection against harmful ingress of water or particulate matter is classified as IPX0
• Optomed Aurora is not intended to be sterilized
• Optomed Aurora is not intended for use in an oxygen rich environment
• Optomed Aurora is classified for continuous operation
Optomed Aurora complies with EU Medical Device Directive 93/42/EEC and its national implementation in the
form of the Finnish Medical Devices Act (629/2010). The valid Declaration of Conformity can be downloaded
at www.optomed.com/support.
 Loading...
Loading...