1. Introduction
1.1 Intended Use
The Tacta® pipette is intended, designed and manufactured for dispensing liquids in a variety of
applications, and to be used in combination with Sartorius Optifit Tips or SafetySpace Filter Tips.
The Sartorius pipette and tip combination, fall within the scope of in-vitro diagnostics, and can
be used as a diagnostic medical device in related applications. Thereby, Tacta® and Sartorius tips
fulfil the relevant demands of the Directive 98/97/EC of the European Parliament.
The Tacta® product range covers a volume range of 0.1 μl to 10 ml. It is recommended that Sartorius Optifit Tips or SafetySpace Filter
Tips are used with Sartorius pipettes to ensure optimum compatibility and performance.
The Sartorius pipette is a general purpose laboratory device that fulfils ISO 9001 and ISO 13485 standards.
Read this user manual carefully before using the pipette for the first time. Additional copies can be downloaded from www.sartorius.
com or hardcopies ordered by email from lhinfo.finland@sartorius.com.
NOTE: Prolonged pipetting can cause Work Related Upper Limb Disorder (WRULD). The manufacturer is not responsible for WRULD or
any related injuries caused by using a pipette.
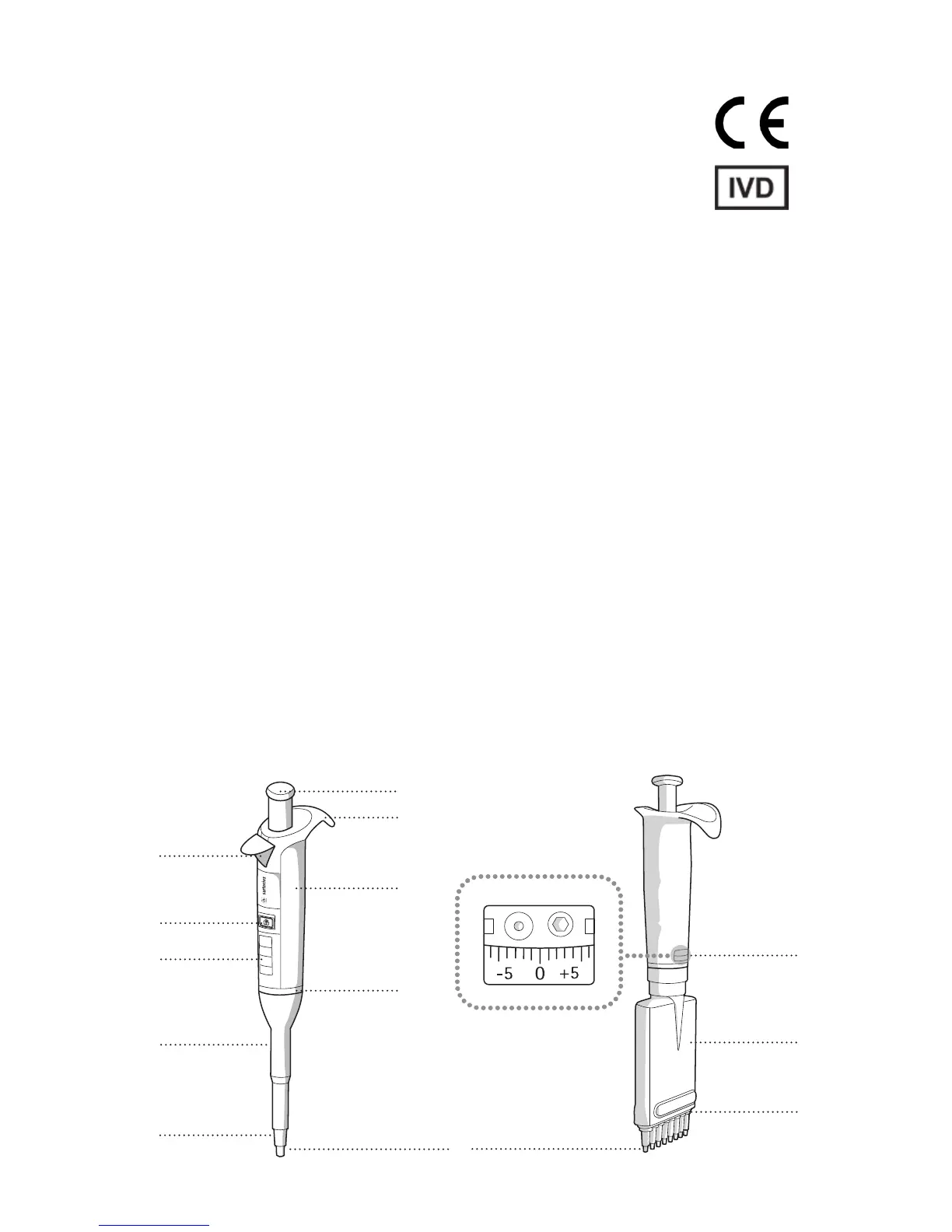
1.2 Pipette Parts and Materials
1. Operating button (polyamide (PA), silicone (SI), stainless steel (SS))
2. Finger support (polypropylene (PP))
3. Tip ejector (PA)
4. Handle (PP)
5. Volume lock (SI)
6. Display (polycarbonate (PC))
7. Tip ejector collar (PP)
8. Tip cone (polyvinylidenefluoride (PVDF) in 3, 10, 20, and 100 μl pipettes, polyetherimide (PEI) in 200 and 300 μl pipettes,
polyphenylenesulphide (PPS) in 1000 μl, 5000 μl, and 10 ml pipettes)
9. Safe-Cone Filter (polyethylene (PE))
10. Adjustment window (PC)
11. Tip cone housing (PA)
12. Tip ejector bar (PA)
13. Stainless steel, corrosion-resistant metal ring (SSt EN 1.4404 / AISI 316L)

 Loading...
Loading...