Approvals
A.1 FDA and IEC marks
SCALANCE XM-400
Operating Instructions, 05/2014, C79000-G8976-C306-03
77
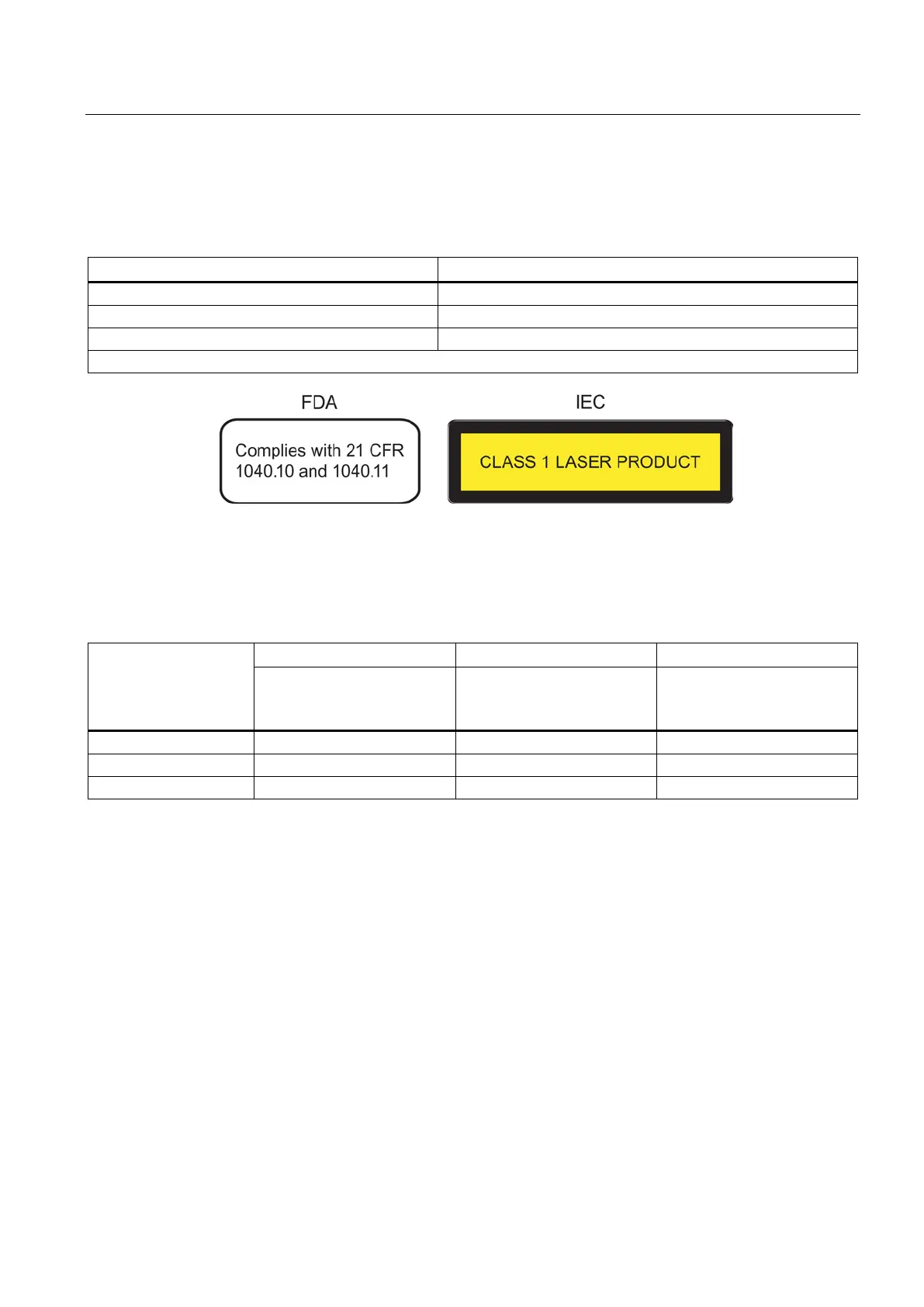
The following devices meet the FDA and IEC requirements listed below:
Fulfills FDA and IEC requirements
Note: With modular devices, the marking is on the extenders and pluggable transceivers.
Figure A-1 FDA and IEC approvals
Mechanical stability (in operation)
IEC 60068-2-6 vibration *
5 - 9 Hz: 3.5 mm
9 - 150 Hz: 1 g
10 - 58 Hz: 0.075 mm
85 - 150 Hz: 1 g
15 g, 11 ms duration
6 shocks per axis
* Note: When installing on an S7-300 or S7-1500 standard rail

 Loading...
Loading...











