51061526 Rev. CVULCAN™ Generator Operations/Service Manual
Introduction / Device Description / Indications for Use / Contraindications / Warnings
Introduction
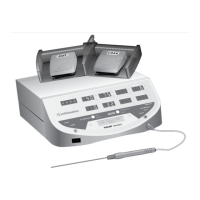
The Smith & Nephew VULCAN™ Generator is designed
to provide finely-controlled radiofrequency (RF) energy
for the coagulation, cutting, ablation, and hemostasis
of soft tissue during a variety of surgical procedures.
The generator control unit is specifically designed for
use with Smith & Nephew temperature-controlled
and power-controlled probes.
Device Description
The generator control unit is a line-voltage powered,
radiofrequency generator capable of delivering up
to 200 watts of power. The generator control unit
includes a neutral electrode monitor (NEM) to monitor
split grounding pad contact quality. It is designed to
be used only with Smith & Nephew temperature-
controlled, cutting, and ablation probes and includes
the following features:
• Two RF output modes: Temperature Control
Mode, where the output power is software-
controlled to maintain a specified tissue
temperature; and Power Control Mode, used for
cutting, ablation, and hemostasis.
• Numerical displays for impedance, set power,
delivered power, set temperature, actual
temperature, set cut, set coagulation, and preset
selections.
• Message display for text messages.
• Indicators for electrode type (monopolar or
bipolar), mode (Temperature Control or Power
Control), stand-by, RF power on, RF output type
(cut or coagulation), fault, and split grounding pad
monitoring. NEM monitors the split grounding
pad connection during surgery, discontinuing
RF delivery if a fault in the NEM monitor circuit is
detected.
• A probe recognition system: the generator control
unit automatically selects the appropriate mode
and preset settings for the probe type.
The Smith & Nephew VULCAN Generator software
can be upgraded at the customer’s location
with a Smith & Nephew supplied PCMCIA card.
For information regarding software upgrades,
please contact your authorized Smith & Nephew
representative.
Indications for Use
The Smith & Nephew VULCAN Generator is
indicated for general surgical purposes, including
orthopedic and arthroscopic applications, for
coagulation, ablation, and hemostasis of soft tissues
in combination with Smith & Nephew temperature-
controlled, cutting, and ablation probes.
Contraindications
There are no known absolute contraindications to the
use of electrosurgery.
The use of the Smith & Nephew VULCAN Generator
and accessories is contraindicated, when, in the
judgment of the physician, an electrosurgical
procedure would be contrary to the best interest of
the patient.
Warnings
• It is the surgeon’s responsibility to be familiar
with the appropriate surgical techniques prior
to use of this device.
• Read these instructions completely prior to
use.
• DANGER: Risk of explosion if used in the
presence of flammable anesthetics, skin
preparation agents, or biointestinal gases.
• Hazardous electrical output. This equipment
should be used only by qualified medical
personnel trained in the use of electrosurgery
devices.
• This device has been tested and verified to
comply with the IEC 60601-2-2. This exceeds
the requirements specified by FCC part 18
for ISM equipment. This device is intended
for operation in a medical facility only. Usage
in a residential environment will likely cause
unacceptable RF interference for which the
user is held responsible.
• The generator control unit must be powered
from a properly grounded 120–240 VAC,
single-phase supply. Excessive risk (leakage
current) may result if this equipment is
connected to other than the manufacturer’s
recommended power distribution system.
• Risk of burns or fire. Do not use near
conductive materials such as metal bed parts,
inner-spring mattresses, etc.
 Loading...
Loading...