EN – 20
Technology
2.1 Technology
2.1.1 Overview
Can also be determined from the serial number, e.g. SN: OR1-2017-XXX refers
to the year 2017.
According to rule 9 of the commission guideline 93/42/EWG, appendix IX, the
OMEGO® System is an active therapeutical Medical Device Class IIa.
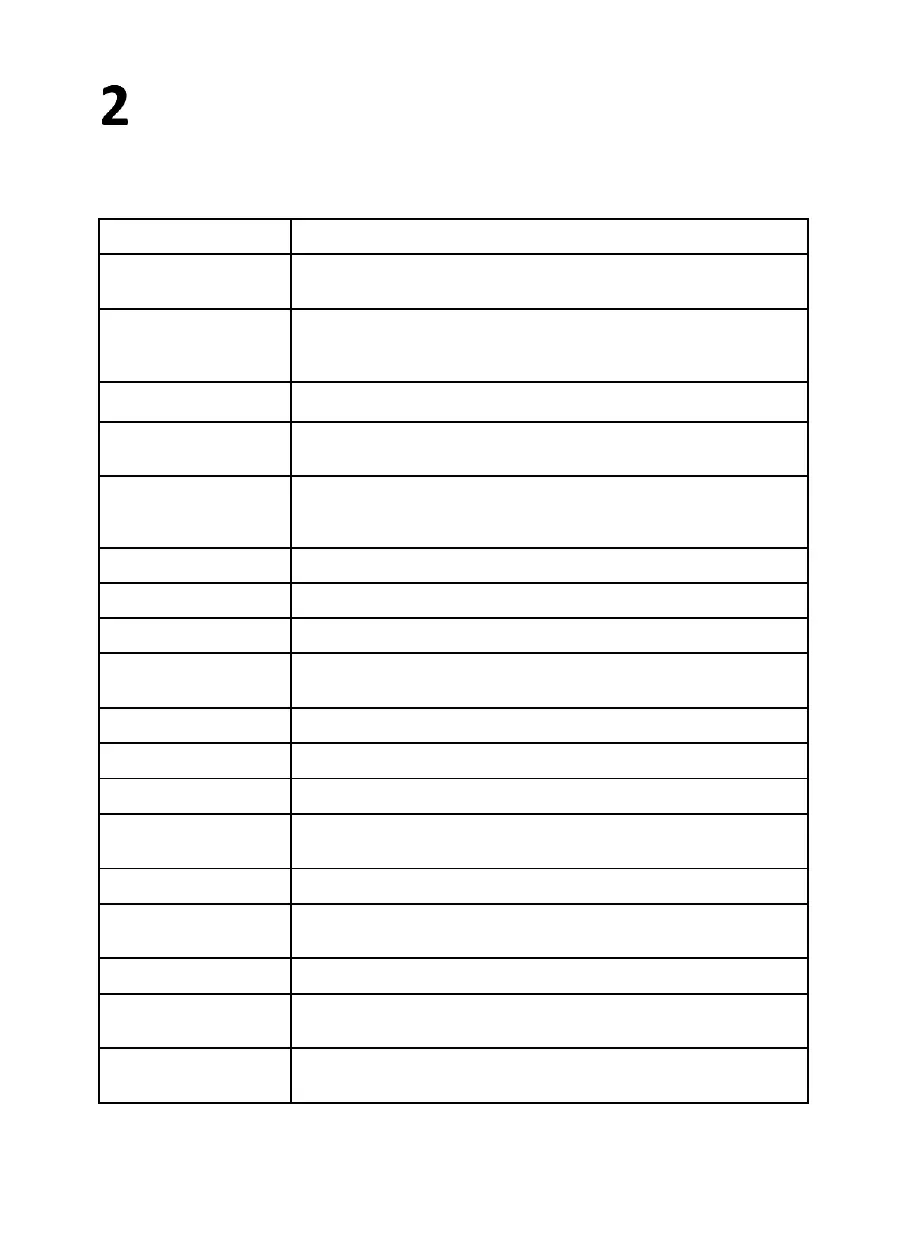
Protection against electric
shock:
Protection class I device – protective grounding
Electromagnetic
compatibility:
Class B device (CISPR 11) The OMEGO® Basic System may only be used in the
living area under the responsibility of a health care professional. EN60601-1-2:
2001, the requirements are fulfilled.
110 – 240V alternating current
Electricity/Power
consumption:
Only connect to supply grids with protective ground wiring.
Secured for all poles (2x T16A L 250V)
Nominal drive performance:
65 Nm unilateral/ 50 Nm bilateral
Measurement range torque
measurement:
Measurement error torque
measurement:
 Loading...
Loading...