1.6. STERILISATION
Every instrument supplied is NOT STERILE and must be sterilised in steam autoclave (max. 135°C) before use, without performing any type of chemical
sterilization.
Sterilization must be performed using appropriate packaging materials, verified during the sterilization process validation.
It is recommended to sterilize in steam autoclave (moist heat) through a cycle with pre-vacuum (forced air removal).
Autoclaves must comply with and be approved and maintained in accordance with the requirements set forth by the Standards EN 13060 (or ANSI/AAMI
ST55), EN ISO 17665-1 and ANSI/AAMI ST79.
Below are the minimum recommended parameters for the sterilization of reusable medical devices, that have been approved in order to ensure a sterility
assurance level (SAL) equal to 10^-6:
• Cycle type: with pre-vacuum (Pre-vac).
• Method: "overkill" moist heat sterilization according to Standard ISO 17665-1.
• Minimum temperature: 134° C (273° F) for heat-resistant materials (instruments and metal handpieces, etc.);121°C (250°F) for thermolabile materials
(rubber items, etc.).
• Minimum exposure time (1): 4 minutes (at 134°C), 20 minutes (at 120°C).
• Minimum drying time (2): defined to ensure compliance with the requirements of Standard EN 13060 (or ANSI/AAMI ST55).
(1) Exposure time: time during which the load and the entire chamber are kept at a temperature higher than the sterilization one.
(2) Drying time: time during which steam is removed from the chamber and the chamber pressure decreases to allow the condensate to evaporate from
the load through a prolonged emptying or thanks to the supply of hot air or other gases then removed.
The drying time varies according to the load configuration and the packaging type and material.
2. DESCRIPTION OF THE EQUIPMENT
2.1. IDENTIFICATION PLATES
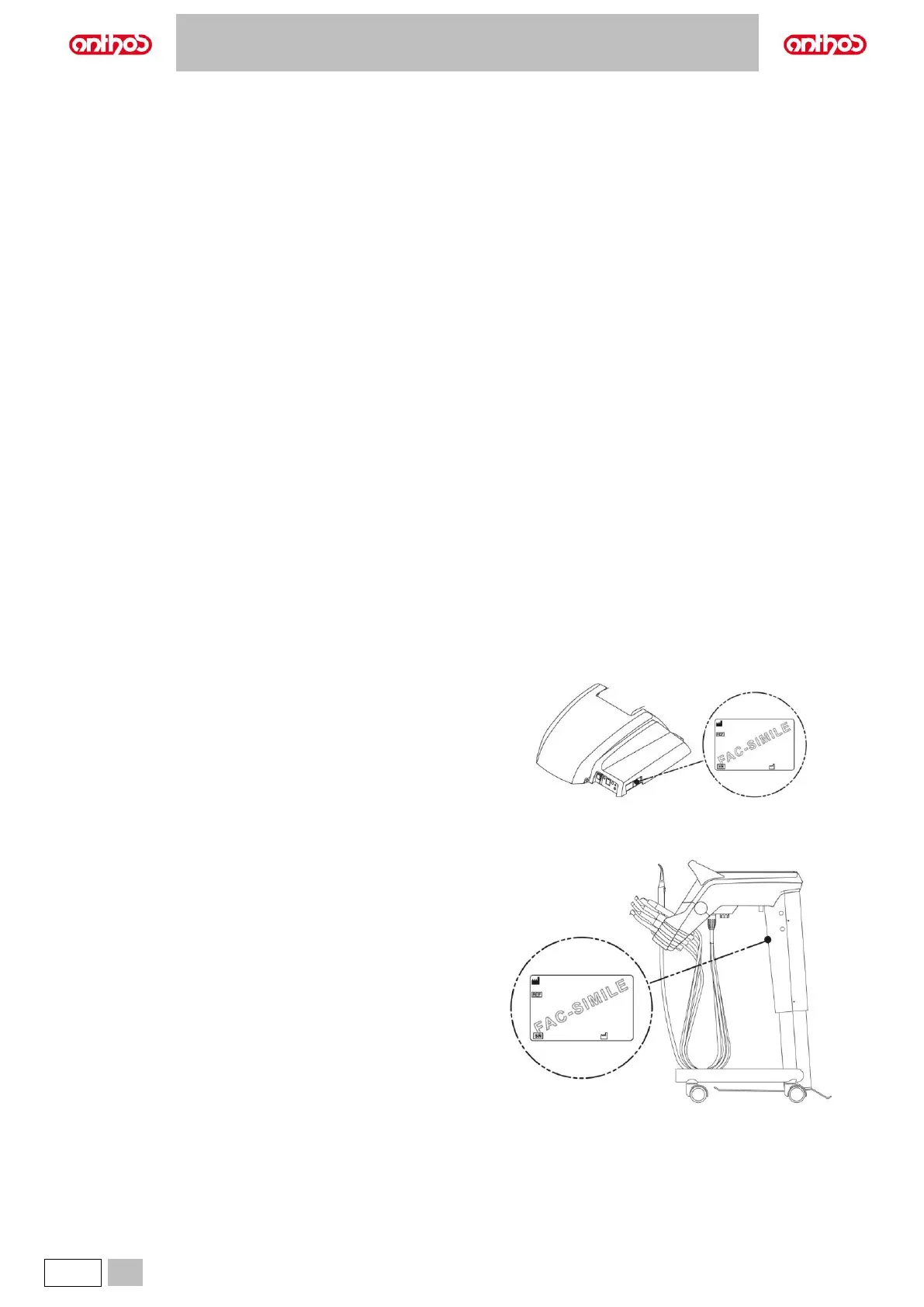 Loading...
Loading...