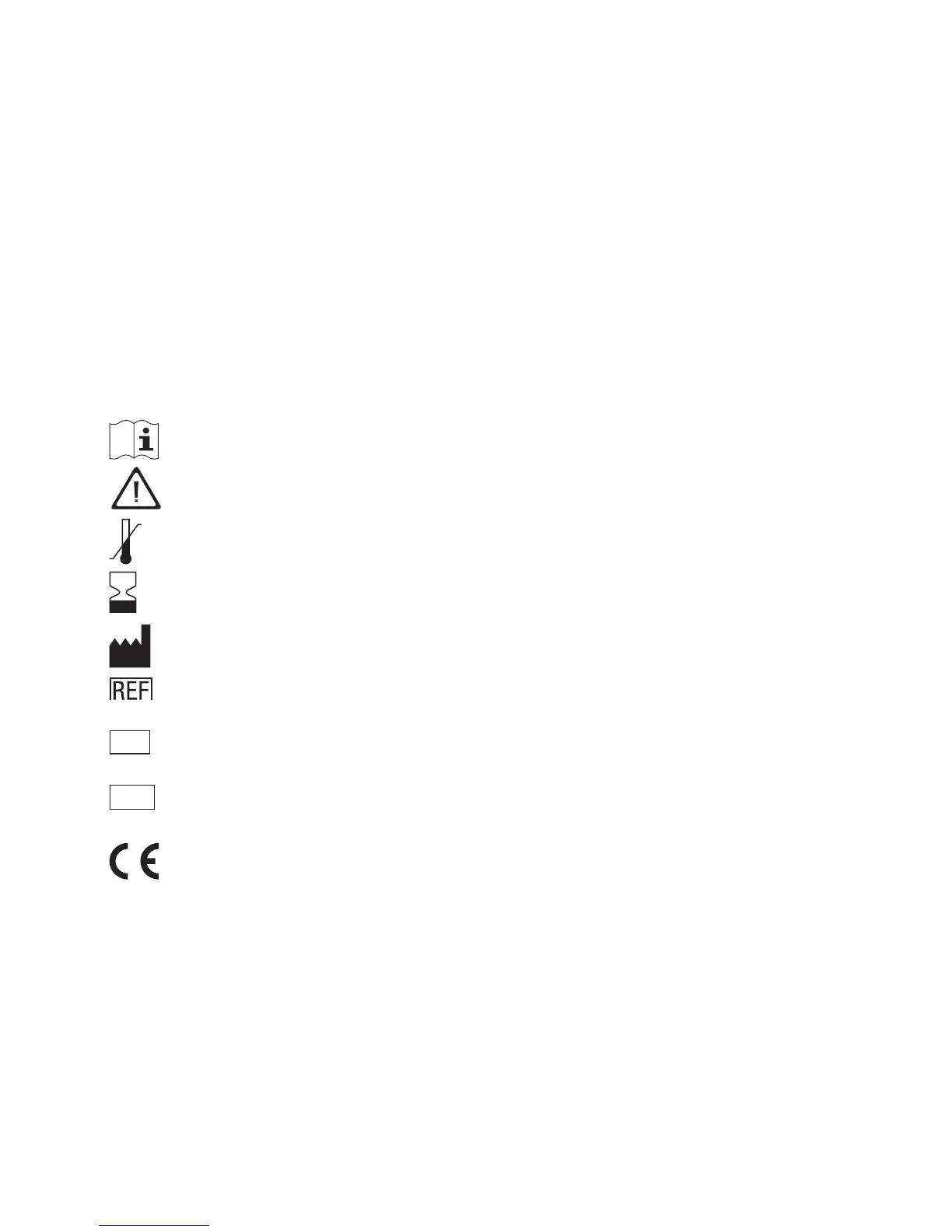
The packaging material, the identification plate of the instrument and the manual may contain the
following symbols or abbreviations which are listed below with their meaning:
Consult instructions for use.
Caution, consult accompanying documents. Refer to safety-related notes in the instruc-
tions for use accompanying this product.
Temperature limitation (store at)
Use by
Manufacturer
Catalog number
In vitro diagnostic medical device.
This product fulfills the requirements of the European Directive 98/79/EC on in vitro
diagnostic medical devices.
 Loading...
Loading...