40
NYHA
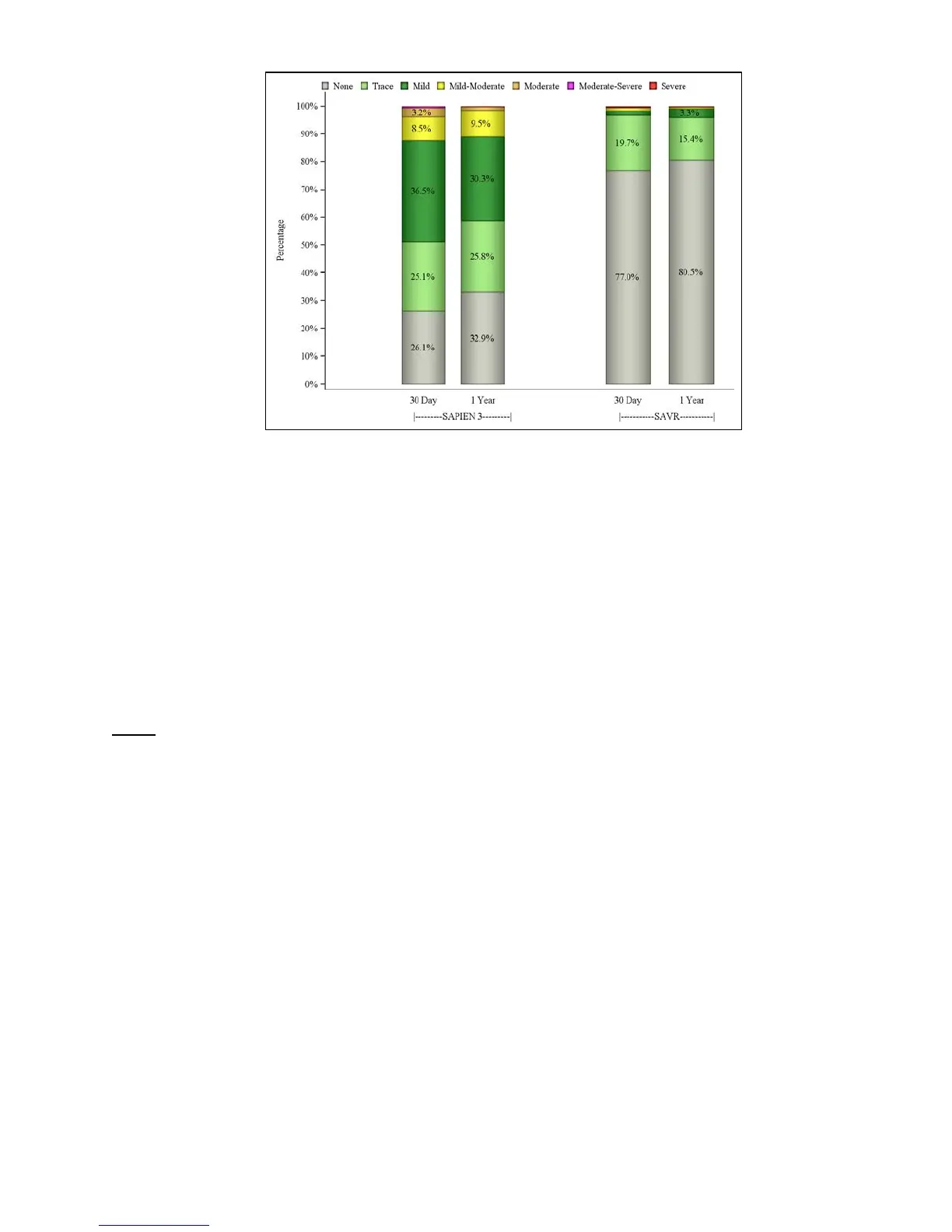
The NYHA classifications by visit are presented in Figure 29. In PIIS3i, 72.6% of the patients
were in NYHA Class III or IV at baseline, which reduced to 6.3% at 30 days and 6.7% at 1 year,
while in PIIA-SAVR, the percentage of patients in NYHA Class III or IV was 76.0% at baseline,
13.6% at 30 days, and 6.7% at 1 year. A side-by-side comparison of the results by access
approach is presented in Figure 30.
 Loading...
Loading...